Herbal medicine Eunkyo-san (Yinqiao-san) for COVID-19: A telemedicine case series
Article information
Abstract
Coronavirus disease 2019 (COVID-19) cases surged across South Korea during the omicron-variant wave. We aimed to report the effectiveness of herbal medicine administered through telemedicine consultations as an adjunctive therapy for COVID-19. Patients with confirmed COVID-19 who were self-isolating at home were provided telephone consultations through a Korean Medicine clinic between January and March 2022. On the basis of their dominant symptoms, the patients were prescribed Eunkyo-san for seven days. Patients were asked to evaluate the severity of their COVID-19 symptoms before and after treatment by numeric rating scale. Of ten patients, more than half of them reported cough, fever, headache, and sore throat on the first telephone consultation. Patients reported that all symptoms related to COVID-19 disappeared completely on the second consultation. No severe adverse events were identified. The results of this case series suggest that Eunkyo-san administration can be a beneficial adjunctive therapy for patients with COVID-19.
Introduction
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) and its variants have emerged1) and spread globally2). The emergence of the B.1.1.529 (omicron) variant was followed by a rapid outbreak across South Korea because of the rapid transmissibility of this variant3,4). Although symptoms related to the omicron variant are likely to be similar or milder than those of the original SARS-CoV-2 and other variants4), the explosive increase in the number of patients with coronavirus disease-19 (COVID-19) during the omicron wave imposed a substantial burden on patients and healthcare providers3,5).
Management of COVID-19 symptoms involves therapeutic approaches from conventional medicine and complementary and alternative medicine (CAM)6,7). Herbal medicine regimens based on CAM principles have demonstrated acceptable effectiveness8). For example, herbal medicines prescribed according to traditional Chinese medicine (TCM) theory resulted in improvements in chest imaging findings and symptoms related to COVID-199). Similarly, observational studies have reported that the herbal medicine Cheongpaebaedok-tang (Quingfeipaidu-tang) was associated with relief from the symptoms of COVID-1910) and reduced in-hospital COVID-19 related mortality11).
Since the South Korean government temporarily allowed telemedicine consultations for conventional medicine and Korean Medicine (KM) early in the pandemic, the COVID-19 KM telemedicine centre was established and prescribed Cheongpaebaedok-tang and other herbal medicines, including Gyeongok-go (Qiongyu-gao), Jaeumbopai-tang (Zhiyinbufei-tang), and Eunkyo-san12). However, since Eunkyo-san was prescribed to only a small proportion (3.0%) of the patients, the effectiveness of this herbal medicine could not be thoroughly determined. Although Eunkyo-san has been suggested to be a potential treatment option for COVID-1913) and the indications of the herbal prescription (sore throat, fever, and cough)13) were similar to the dominant symptoms of COVID-19 during the omicron-variant surge14), evidence supporting the clinical effectiveness of Eunkyo-san is lacking. Therefore, this case series aimed to report improvements in the symptoms of COVID-19 after Eunkyo-san administration via telemedicine.
Case
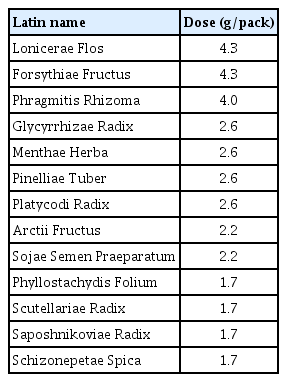
Since the temporary allowance of telemedicine in South Korea due to the pandemic15), patients self-isolating at home after confirmation of COVID-19 have been offered telephone consultation by a Korean Medicine Doctor (KMD) at Gyeongbuk KM clinic. Because the patients had been treated for their health problems at this KM clinic before the pandemic, the KMD prescribed herbal medicine via telemedicine while considering the patients’ current COVID-19 symptoms as well as their medical history and/or underlying conditions. Details regarding COVID-19, including vaccination and current COVID-19 medication, were also evaluated. The symptoms of the patients during the omicron-variant wave were similar to the indications of Eunkyo-san, ie., sore throat, fever, and cough13). All of the patients were prescribed the herbal medicine and advised to follow a dosing regimen in which a total of six packs of decoction (150 mL/pack) were orally administered six times daily (one pack per time: 1 hour after breakfast, before lunch, after lunch, before dinner, after dinner, and before sleep) for the first three days, followed by oral administration of a total of three packs of decoction (150 mL/pack) three times daily (one pack per time: 1 hour after respective meals) for the next four days. The medicinal herbs used in Eunkyo-san are listed in Table 1. Patients were advised to complete the Eunkyo-san prescription even if the COVID-19 symptoms disappeared during administration. Conventional treatment was allowed for existing conditions and/or for relieving the symptoms of COVID-19.
Evaluation methods and treatment progress
1. Evaluation methods
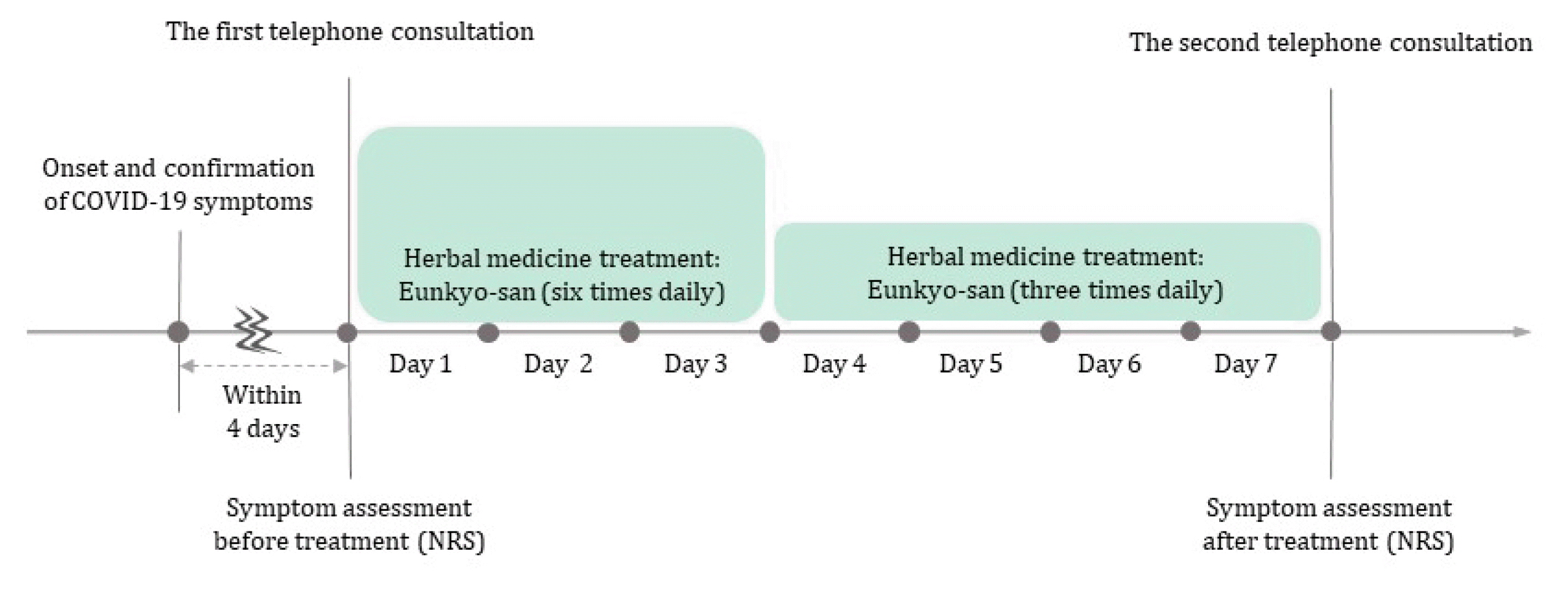
On the first and second telephone consultations, patients assessed the severity of 15 symptoms attributable to COVID-19, such as ageusia, anosmia, chest congestion, cough, fever, and headache, using numeric rating scale (NRS) before and after the oral administration of the herbal decoction. A score of 0 indicated no symptoms, and 10 indicated the worst symptom imaginable. Adverse events (AEs) were recorded verbatim. The period between telephone consultations were mostly seven days. A schematic representation of this case series is shown in Fig. 1.
This study was approved by the Institutional Review Board of the Korea Institute of Oriental Medicine (I-2207/007-002). Patients who provided voluntary electronic informed consent were included in this analysis. Statistical analysis was performed using R version 4.1.3 (R Development Core Team 2022). Categorical variables were expressed as numbers and percentages, and continuous variables were described as median (range). Differences in the NRS scores for acute COVID symptoms before and after oral administration of Eunkyo-san were compared using a paired-sample Wilcoxon test. The results were considered statistically significant when the p-value was <0.05.
2. Treatment progress
1) Characteristics of the patients
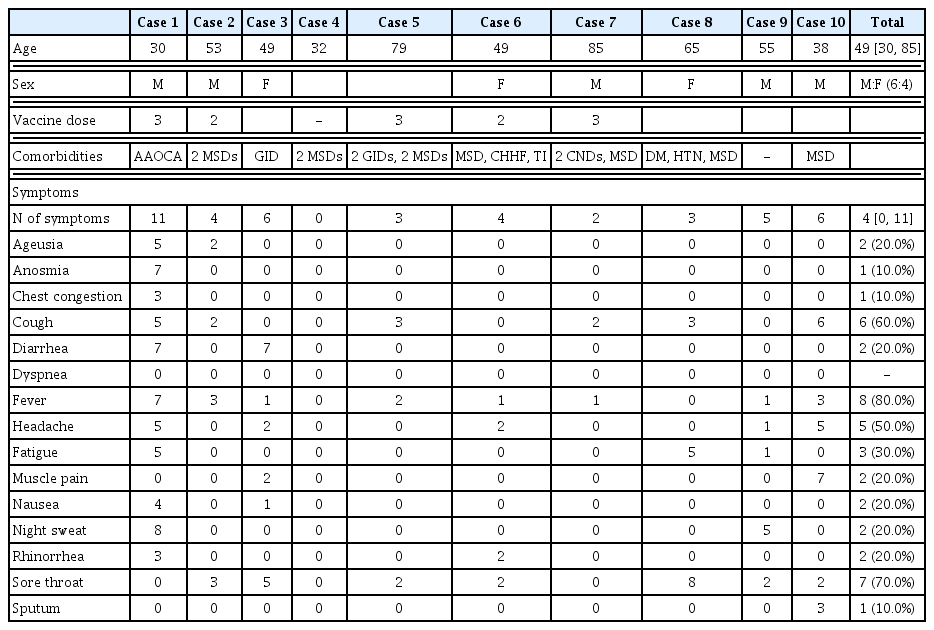
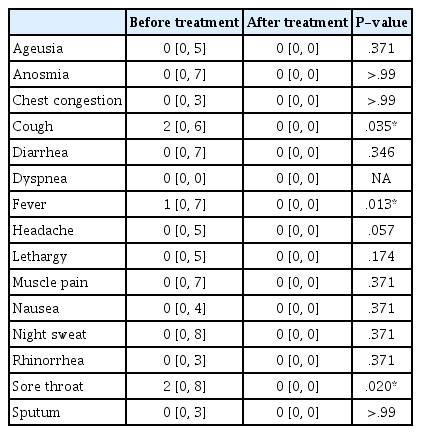
Between January and March 2022, 10 patients with polymerase chain reaction (PCR)-confirmed COVID-19 were offered telephone consultations through the KM clinic and provided electronic informed consent. The median period between the onset of symptoms and telephone consultation was 1 day (range: 1–4 days), and the median interval between COVID-19 confirmation and consultation was 1 day (range: 0–3 days). The median age of the patients was 49 years (range: 30–85 years); more than half of the patients were male (6 patients). One of the 10 patients had not received COVID-19 vaccination, while the others had received two (2 patients) or three doses (7 patients) of the vaccine (Table 2). Musculoskeletal disorders were the most frequent comorbidity, and almost half of the patients (4 patients) had more than three comorbidities. Among the COVID-19 symptoms, cough, fever, and sore throat were dominant, with median symptom severities of 2 (cough), 1 (fever), and 2 (sore throat), respectively (Table 3, Fig. 2). All COVID 19 symptoms disappeared after 7-day administration of Eunkyo-san. Statistical analysis indicated that cough, fever, and sore throat improved significantly after herbal medicine treatment. Neither severe AEs nor COVID-19-related hospitalisations were reported.
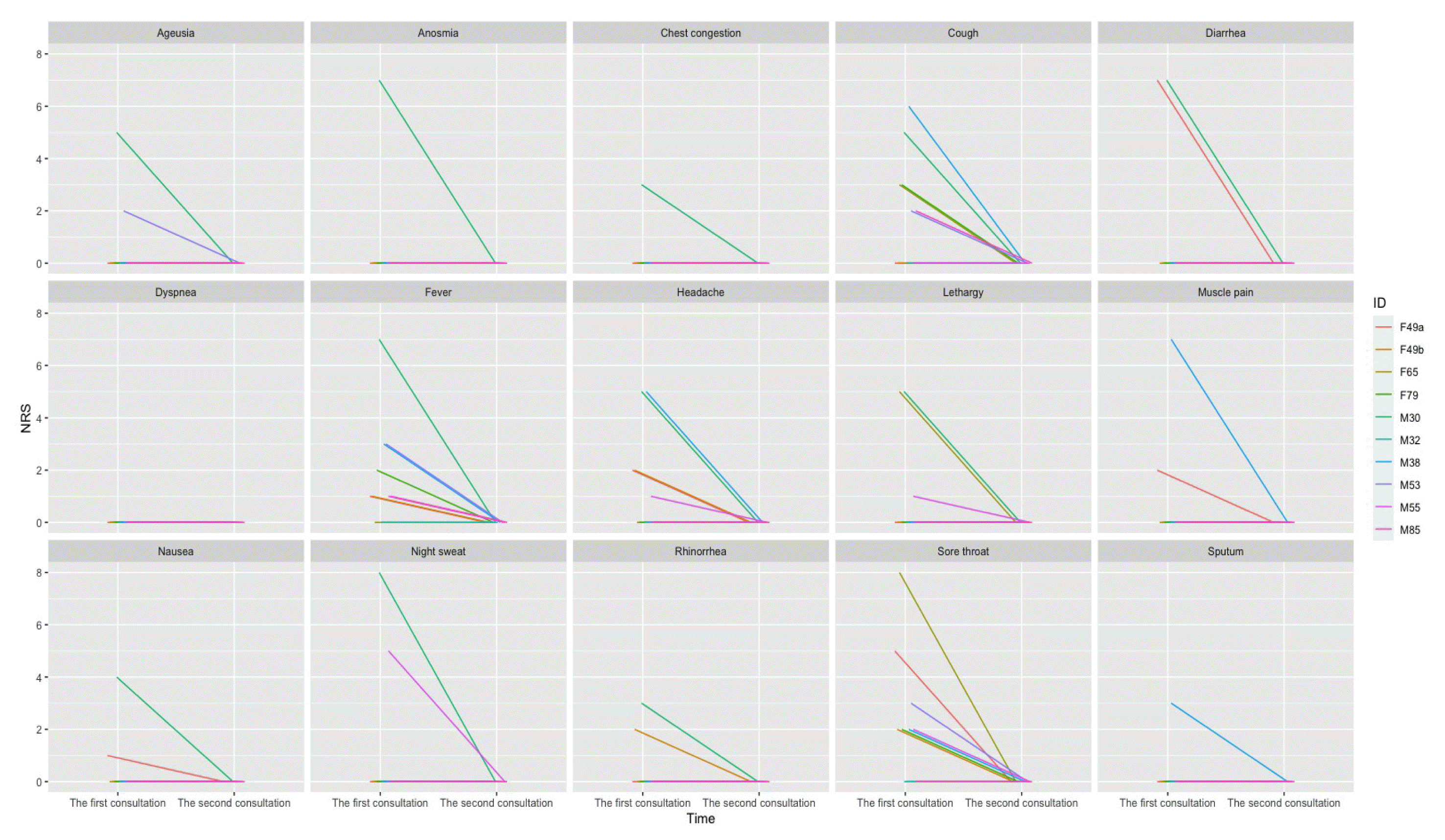
Schematic visualization of COVID-19 symptoms before and after Eunkyo-san administration
IDs indicate sex (M: male F: female) and age of respective patients.
Case 1 involved a 30-year-old male patient with congenital heart disease (anomalous aortic origin of the coronary artery). Although he was vaccinated (three doses) and was the youngest among the 10 patients, he had 11 of the COVID-19 symptoms investigated (ageusia, anosmia, chest congestion, cough, diarrhoea, fever, headache, fatigue, nausea, night sweats, and rhinorrhea), and the median symptom severity measured with NRS was 5, which was the highest among the 10 patients. His symptoms were relieved after three days of Eunkyo-san administration. The COVID-19 symptoms in case 2 were considered mild-to-moderate, since the patient’s maximum symptom severity score was 4 16,17). The patient’s symptoms resolved after four days of herbal medicine treatment. Case 3 involved a 49-year-old female patient who reported severe diarrhoea, moderate sore throat, mild fever, muscle pain, headache, and nausea during the first telephone consultation. Although all clinical symptoms except diarrhoea were resolved in a day, the diarrhoea lasted for 7 days and resolved spontaneously. The patient in case 4, who was asymptomatic throughout the treatment period, received a telephone consultation immediately after COVID-19 confirmation because of his desire to prevent the spread of COVID-19 in his community.
Cases 5, 7, and 8 involved elderly patients with multiple comorbidities (case 5: dyspepsia, irritable bowel syndrome, cervical and lumbar herniated intervertebral discs; case 7: dementia, cerebral infarction, and osteoporosis; case 8: diabetes mellitus, hypertension, lumbar herniated intervertebral discs, and hyperhidrosis). The patient in case 8 reported that her sore throat worsened and it felt like dying before the initiation of treatment. Although she experienced heartburn and stomachache on the fifth day after herbal medicine treatment, her symptoms disappeared spontaneously on the seventh day.
Case 6 involved a 49-year-old female patient with more than three underlying diseases (lumbar herniated intervertebral disc, tinnitus, and cold hypersensitivity in the hands and feet) who developed a severe rash (circular shape with reddish bumps) during the treatment period. The rash resolved within one day without any specific treatment. Cases 9 and 10 involved middle-aged male patients who reported resolution of their COVID-19 related symptoms, although their initial symptoms and clinical courses were different. In case 9, the patient had mild-to-moderate symptoms considering the NRS value, which ranged between 0 and 5 before the initiation of herbal medicine, and the patient recovered from all COVID-19 symptoms within a day. On the other hand, the patient in case 10, who had moderate-to-severe cough and muscle pain before treatment, felt that his symptoms temporarily worsened following the 3-day administration. He rapidly recovered and his symptoms vanished on the sixth day.
3. patient perspective
All patients reported that they were satisfied with the results of herbal medicine treatment, that is, the resolution of their acute COVID-19 symptoms. They reported that more frequent administration of Eunkyo-san during the first three days was acceptable, even though the number of oral administrations on these three days was twice the usual number. In a comparison of telephone consultations with face-to-face consultations, they reported satisfaction with the remote consultations offered by the KM clinic because it was available during self-isolation after COVID-19 confirmation, unlike face-to-face consultations.
Discussion
Ten COVID-19 patients received telephone consultations at one KM clinic and were prescribed the herbal medicine for 7 days as an adjunct to conventional treatment during the omicron wave. Since the patients’ NRS scores improved after the administration of the drug and the safety profile and mode of consultation were acceptable to the patients, Eunkyo-san has the potential to be a treatment option for self-isolating patients with COVID-19 symptoms of sore throat, cough, and fever.
A recent study evaluating other herbal medicine therapies such as Cheongpaebaedok-tang directly demonstrated the efficacy of these therapies against COVID-1918). Some recent studies have suggested that Eunkyo-san can improve infectious diseases other than COVID-1919) or examined the possible treatment mechanisms underlying the effect of herbal medicine and suggested its provisional effectiveness against COVID-1913,20); However, direct evidence on the clinical effectiveness of Eunkyo-san in patients with COVID-19 is lacking. To the best of our knowledge, this is the first telemedicine case series to explore the effectiveness of Eunkyo-san on the symptoms of acute COVID-19.
Eunkyo-san is prescribed for the onset of febrile diseases and respiratory infections21,22). The effectiveness of herbal medicine treatment has been tested for several diseases, including upper respiratory tract infections, common cold, and H1N1 influenza, and its clinical superiority over control interventions has been shown to vary across studies. The AEs reported in this case series were not severe, and most of them were gastrointestinal disorders, such as diarrhoea, heartburn, and stomachache. While this is consistent with the results of a previous randomised clinical trial that reported gastrointestinal AEs in participants of both groups (Eunkyo-san and placebo group)23), a causal association between herbal medicine and AEs can be established with more evidence. During the 7-day administration period, patients were requested to take Eunkyo-san twice frequently for the first three days because this method can enhance the effectiveness of the herbal medicine 22).
This case series had some limitations. First, the lack of generalisability was problematic. This case series only reported the findings for patients who received telephone consultations through a KM clinic. The clinical characteristics of the patients in this study, however, were concordant with those of patients admitted to tertiary hospitals in that they had mild symptoms14). Second, this telemedicine case series measured the effectiveness of herbal medicine only using patient-reported outcomes and did not collect follow-up data. The NRS is a subjective outcome measure that can be influenced by outcome assessors’ expectations24). Because all of the participants had been treated for existing health problems before the pandemic and were assumed to be favourable to KM, this may have affected their expectations and the treatment effectiveness assessed by patient-reported outcome measures. Since this case series describes the experiences from one KM primary clinic25), objective outcome measures that can be easily evaluated by primary clinic practitioners in primary care clinics are needed in future case reports or case series. Third, given that this study was a case series in a primary care setting and conventional medicine treatment was allowed for relieving the symptoms of COVID-19, the causal relationship between Eunkyo-san and possible benefits (improvement in COVID-19 symptoms) and harm (gastrointestinal AEs, such as diarrhoea, heartburn, and stomachache) cannot be firmly established. More robust study designs, such as prospective cohort studies or randomised controlled trials, are needed to determine the effectiveness of herbal medicine treatment alone or as an adjunct to conventional medicine approaches.
On the basis of the results of this case series, KMDs can prescribe Eunkyo-san as adjunctive therapy when COVID-19 patients present symptoms of sore throat, fever, and cough without gastrointestinal symptoms such as diarrhoea, heartburn, and stomachache, until high-quality evidence for the herbal medicine approach is established.
Conclusions
The results of this telemedicine case series suggest that Eunkyo-san could be a therapeutic option for acute COVID-19. Further studies are warranted to validate the effects of Eunkyo-san on COVID-19 and other infectious diseases.
Acknowledgement
This study was supported by the Korea Institute of Oriental Medicine (KSN1823211) and supported by Policy support project of Korean Medicine for the infectious disease, funded by the Ministry of Health and Welfare; This work was supported by Dong-eui University Grant.(202301270001)
Notes
Competing interest statement
The authors declare no conflict of interest.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.