Management of asymptomatic to mild COVID-19 patients with Cheongpebaedok-tang on the telemedical basis: A retrospective observational case series
Article information
Abstract
Objectives
This retrospective observational study aimed to investigate the efficacy and safety of Cheongpebaedok-tang, a traditional Korean herbal medicine, provided via telemedicine to patients with asymptomatic to mild COVID-19 in Korea.
Methods
From February to April 2020, a retrospective analysis investigated COVID-19 patients treated via Korean telemedicine. The study involved asymptomatic to mild cases receiving Cheongpebaedok-tang more than three times, along with continuous Korean medicine care in convalescence. Diagnoses and treatment adhered to the telemedicine guidelines of the Association of Korean Medicine, with varied Cheongpebaedok-tang prescriptions based on symptom severity. Symptom evaluation involved a detailed assessment using a 15-item tool at initial and final sessions.
Results
The study included 27 patients, with a mean age of 48.7 ± 2.3 years (mean ± standard error). Patients began self-administering oral Cheongpebaedok-tang for an average of 19.4 ± 1.8 days after the date of COVID-19 diagnosis confirmation and continued the medication for 15.8 ± 1.2 days. The reported side effects of the Cheongpebaedok-tang included palpitations (11.1%), insomnia (7.4%), dizziness (3.7%), and diarrhea (3.7%). All side effects disappeared after adjusting the prescription according to standard treatment guidelines. The occurrence of all COVID-19-related adverse symptoms, except fatigue and myalgia, decreased. Fatigue was the most common chronic symptom persisting after 6 months (51.9%), followed by ocular symptoms (37.0%) and sore throat (22.2%).
Conclusions
This study implies Cheongpebaedok-tang may offer a potentially safe, symptom-alleviating approach for managing mild COVID-19 cases via telemedicine, although further comprehensive research is warranted.
Introduction
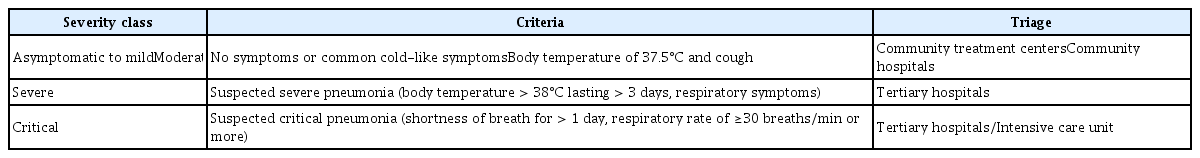
Coronavirus disease 2019 (COVID-19) started spreading in Wuhan City, Hubei Province, China at the end of 2019 and was declared a global pandemic by the World Health Organization on March 11, 2020.1) After the first confirmed COVID-19 case in the Daegu region of Gyeongsangbuk-do on February 18, 2020, the number of patients with COVID-19 exponentially increased in the Republic of Korea, resulting in an epidemic with 5,000 confirmed cases by March 7, 2020. The Korea Centers for Disease Control and Prevention operated the “Regional Risk Stratification and Triage System” in Daegu. The severity class was divided into asymptomatic, mild, moderate, severe, and critical by doctors who interviewed patients by phone (Table 1).2)
The Association of Korean Medicine (AKOM) operates the telemedicine center of Korean medicine, targeting patients with asymptomatic to mild and moderate COVID-19. The AKOM has published telemedicine guidelines for COVID-19 management which present a treatment algorithm3) based on previously published Korean medicine and traditional Chinese medicine treatment guidelines, such as those in COVID-19 Korean Medicine Clinical Guidance (2nd ed.)4), the Clinical Guidelines on COVID-19 Korean Medicine5), and the Diagnosis and Treatment Protocol for COVID-19 (Trial Version 7).6) An average of 15 doctors provided treatment at the telemedicine center for an average of 192 patients per day from March 3, 2020 to April 12, 2020; as of April 13, 2020 the proportion of patients using telemedicine centers among all COVID-19 confirmed cases was 16.53% of all patients in Korea.7)
Cheongpebaedok-tang (Qingfei Paidu decoction in China) is the main herbal medicine recommended for the treatment of all stages of COVID-19 by the National Health Commission of the People’s Republic of China.8) It is one of the most effective herbal medicines for treating COVID-19 and is strongly recommended by most traditional Korean medicine guidelines.4–6) Therefore, Cheongpebaedok-tang was recommended as a basic prescription for patients with confirmed COVID-19 in the telemedicine guidelines.3) This study investigated the efficacy and safety of telemedical management using Korean medicine centered on Cheongpebaedok-tang for treating patients with asymptomatic to mild COVID-19.
Methods
1. Study design and patients
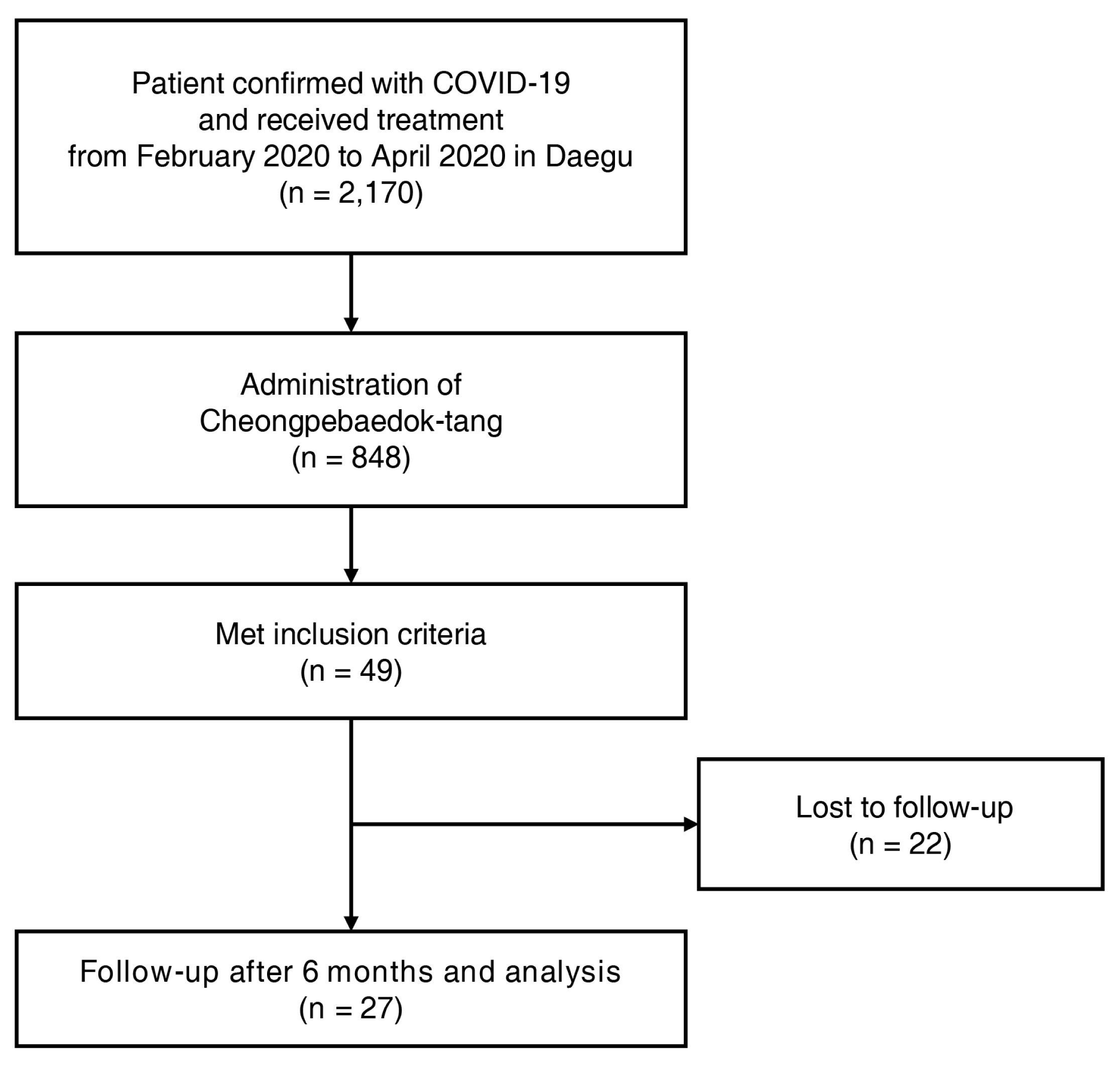
A retrospective analysis of the medical records of patients who were diagnosed with COVID-19 in Daegu and treated using herbal medicines at the telemedicine center of Korean medicine from February to April 2020 was performed. The inclusion criteria were as follows: patients (1) with asymptomatic to mild confirmed COVID-19; (2) who had Cheongpebaedok-tang administered more than three times; and (3) who were continuously managed using Korean medicine in the convalescent period. Patients who could not be followed up were excluded as determined by a follow-up telephone interview after six months.
The Korean medicine diagnoses of COVID-19 symptoms and prescriptions were decided by phone by Korean medicine doctors based on the telemedicine guidelines of the AKOM. Participants for Korean medicine treatment were limited to those who had experience in self-isolation. Telephone counseling for patients in isolation was conducted at 3-day intervals, and 3 days’ worth of medication was administered. The treatment of patients who were convalescing, were released from isolation, and had no symptoms was terminated after physicians prescribed 5 days’ worth of medication. The total Korean medicine treatment period was based on 3 weeks from the date of initial administration (2 weeks for patients released from isolation and 5 days for asymptomatic patients after release) and was shortened or extended at the discretion of the Korean medicine doctor depending on the condition of the patient.3)
2. Cheongpebaedok-tang
Cheongpebaedok-tang was divided into two prescriptions: Cheongpebaedok-tang 1 was the prescription suggested by the Chinese guidelines,8) and Cheongpebaedok-tang 2 was Cheongpebaedok -tang 1 with ephedra excluded. Cheongpebaedok-tang 1 was used as a first-line treatment for patients with mild symptoms among the COVID-19 confirmed cases. Cheongpebaedok-tang 2 was prescribed to patients who were asymptomatic or to patients with symptoms who were concerned about the side effects of ephedra.3)
3. COVID-19-related symptom severity
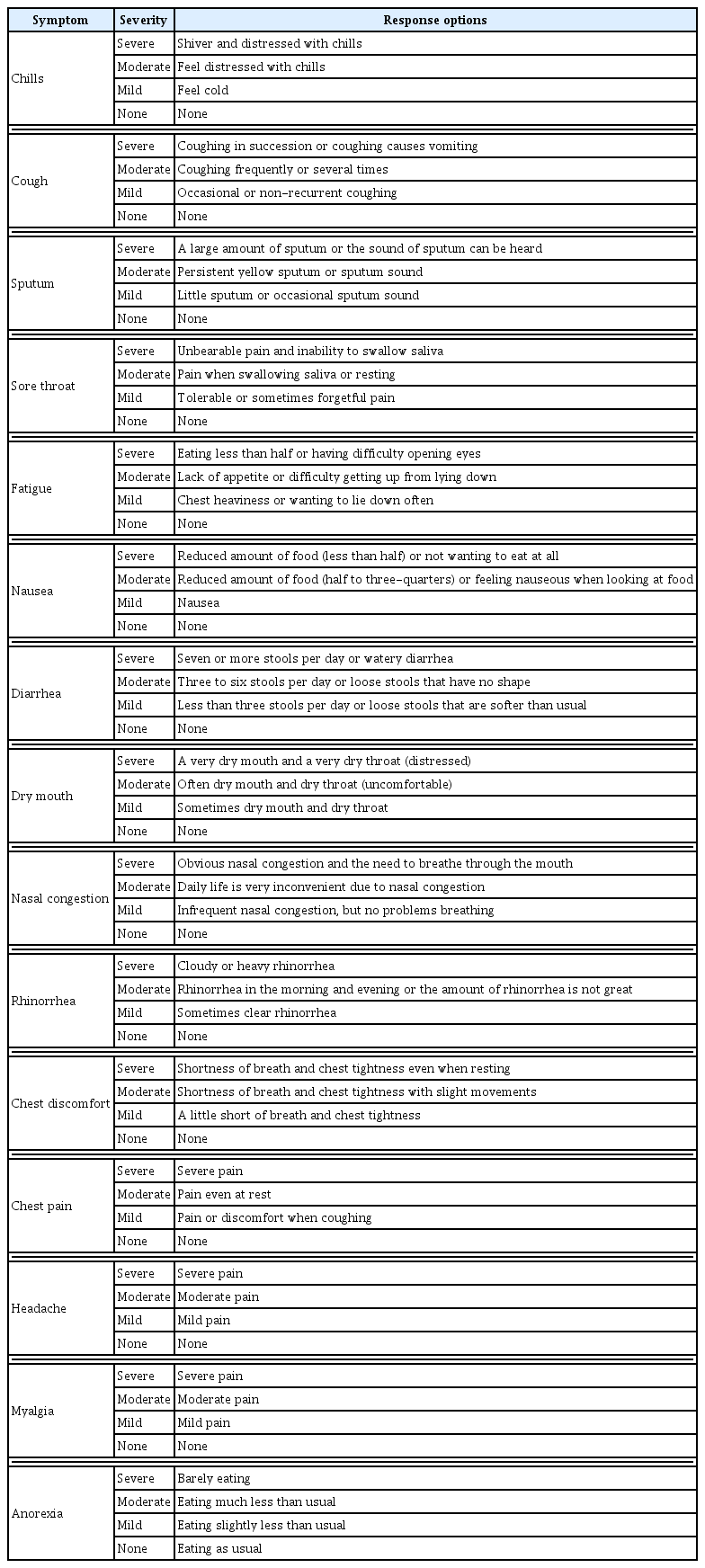
At the first and last treatment sessions, a Korean medicine doctor evaluated COVID-19 -related symptoms to determine the severity. The evaluation tool consists of 15 symptom items, each of which was evaluated as none, mild, moderate, or severe (Table 2).
4. Statistical analysis
Continuous variables were expressed as mean ± standard error (SE), and categorical variables were expressed as frequencies (%). The statistical methods of this study were reviewed by Ojin Kwon from KM Science Research Division, Korea Institute of Oriental Medicine.
5. Ethical considerations
This study analyzed the medical records provided by AKOM. This study was approved by the Institutional Review Board (IRB) of Kyung Hee University Korean Medicine Hospital. The IRB approved a waiver of the consent for this study (KOMCIRB 2021-10-007).
Results
1. Patient characteristics
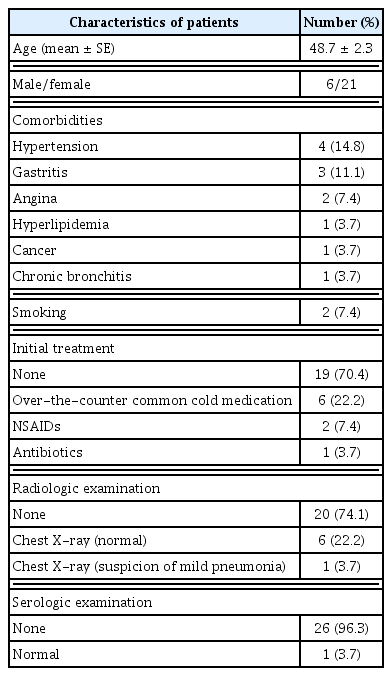
Twenty-seven patients were included in this analysis (Figure 1). The mean patient age was 48.7 ± 2.3 years. The comorbidities included hypertension in four patients (14.8%), gastritis in three patients (11.1%), angina in two patients (7.4%), and hyperlipidemia, cancer, and chronic bronchitis in one patient each (3.7%). Two (7.4%) patients had a history of smoking. The initial treatment was no treatment in 19 patients (70.4%), over-the-counter cold medicines in six patients (22.2%), non-steroidal anti-inflammatory drugs in two patients (7.4%), and antibiotics in one patient (3.7%). During the initial evaluation of patients, radiological evaluation was not performed in 20 patients (74.1%). Among seven patients who underwent chest radiography, six patients (22.2%) had normal findings, and mild pneumonia was suspected in one patient (3.7%). During the initial evaluation, serological evaluation was not performed in 26 patients (96.3%), and one serologically-evaluated patient showed values that were within the normal reference range (Table 3).
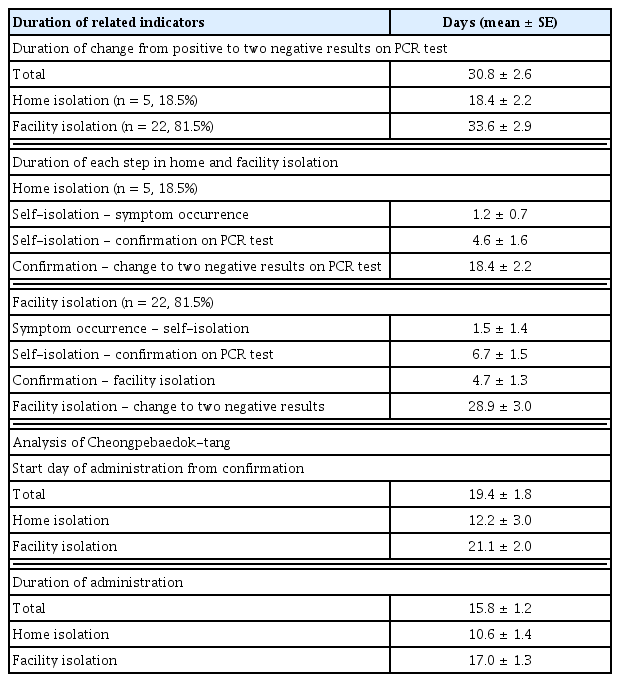
2. Duration of related indicators in patients with COVID-19
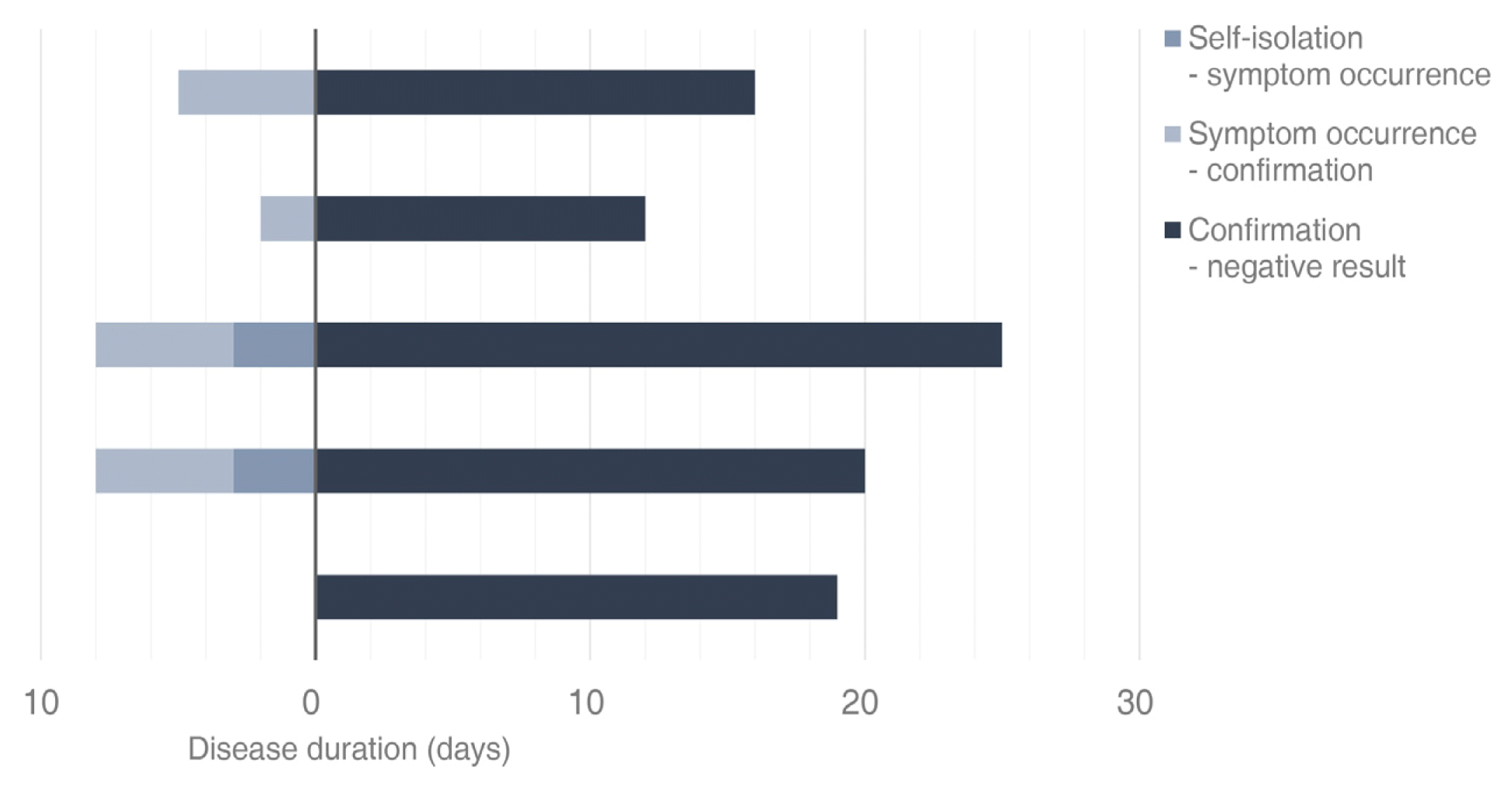
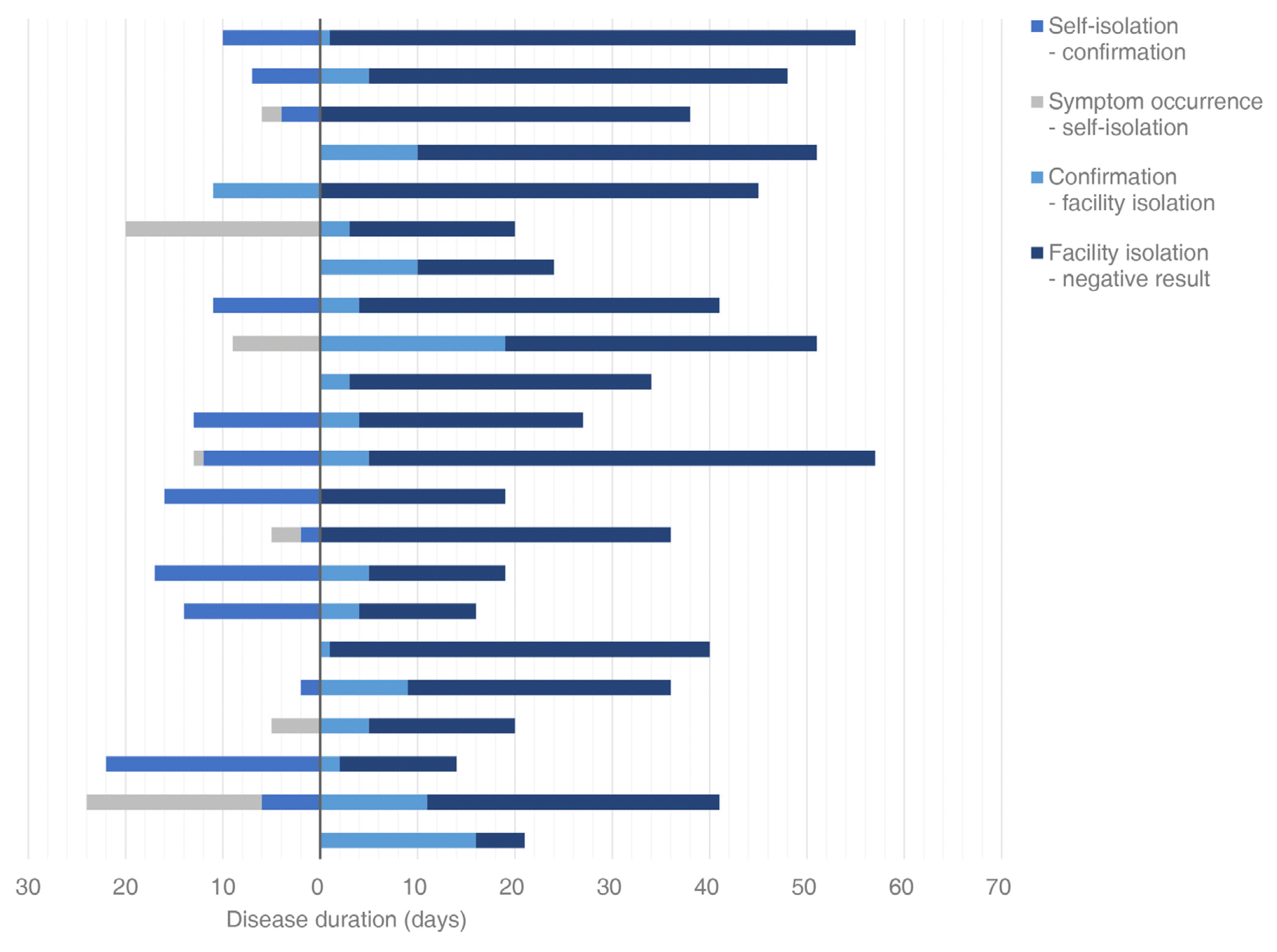
The duration of change from positive to two negative results on the polymerase chain reaction (PCR) test was 30.8 ± 2.6 days. When classified according to the type of isolation, results revealed 18.4 ± 2.2 days in five patients who were in home isolation (19%) and 33.6 ± 2.9 days in 22 patients who were in facility isolation (81%). In patients who were isolated at home, 1.2 ± 0.7 days passed from self-isolation to the onset of symptoms, 4.6 ± 1.6 days from self-isolation to confirmation of COVID-19 on the PCR test, and 18.4 ± 2.2 days from confirmation of two negative results on the PCR test. In patients who were isolated in facilities, 1.5 ± 1.4 days passed from onset of symptoms to self-isolation, 6.7 ± 1.5 days from self-isolation to confirmation of COVID-19 on the PCR test, 4.7 ± 1.3 days from confirmation to isolation in facilities, and 28.9 ± 3.0 days from facility isolation to two negative results on PCR tests (Table 4, Figures 2 and 3).
3. Duration and side effects analysis of Cheongpebaedok-tang
The interval between the date a COVID-19 diagnosis was confirmed and the start date of administration of Cheongpebaedok-tang was 19.4 ± 1.8 days: 12.2 ± 3.0 days for patients who were isolated at home and 21.1 ± 2.0 days for patients who were isolated in facilities. The average administration duration was 15.8 ± 1.2 days: 10.6 ± 1.4 days for patients in home isolation and 17.0 ± 1.3 days for those in facility isolation (Table 4). Regarding the side effects of Cheongpebaedok-tang, palpitations were reported in three patients (11.1%), insomnia in two patients (7.4%), dizziness in one patient (3.7%), and diarrhea in one patient (3.7%). All side effects were resolved upon changing the medication from Cheongpebaedok-tang 1 to 2 or when the number of doses was reduced in accordance with telemedicine guidelines.
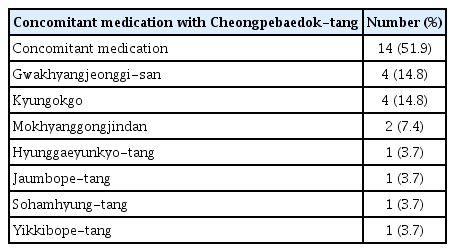
4. Concomitant medication with Cheongpebaedok-tang
Fourteen patients (51.9%) self-administered other herbal medicines concomitantly, including four patients (14.8%) taking Gwakhyangjeonggi-san, four patients (14.8%) taking Kyungokgo, two patients (7.4%) taking Mokhyanggongjindan, and one patient each (3.7%) taking Hyunggaeyunkyo-tang, Jaumbope-tang, Sohamhyung-tang, and Yikkibope -tang (Table 5).
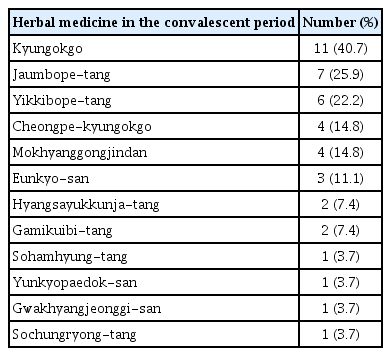
5. Herbal medicine in the convalescent period
Regarding the herbal medicine used in the convalescent period, Kyungokgo was the most commonly used medicine in 11 patients (40.7%), followed by Jaumbope-tang in seven patients (25.9%), Yikkibope-tang in six patients (22.2%), Cheongpe-kyungokgo in four patients (14.8%), Mokhyanggongjindan in four patients (14.8%), Eunkyo-san in three patients (11.1%), Hyangsayukkunja-tang in two patients (7.4%), Gamikuibi-tang in two patients (7.4%), and Sohamhyung-tang, Yunkyopaedok-san, Gwakhyangjeonggi-san, and Sochungryong-tang in one patient each (3.7%) (Table 6).
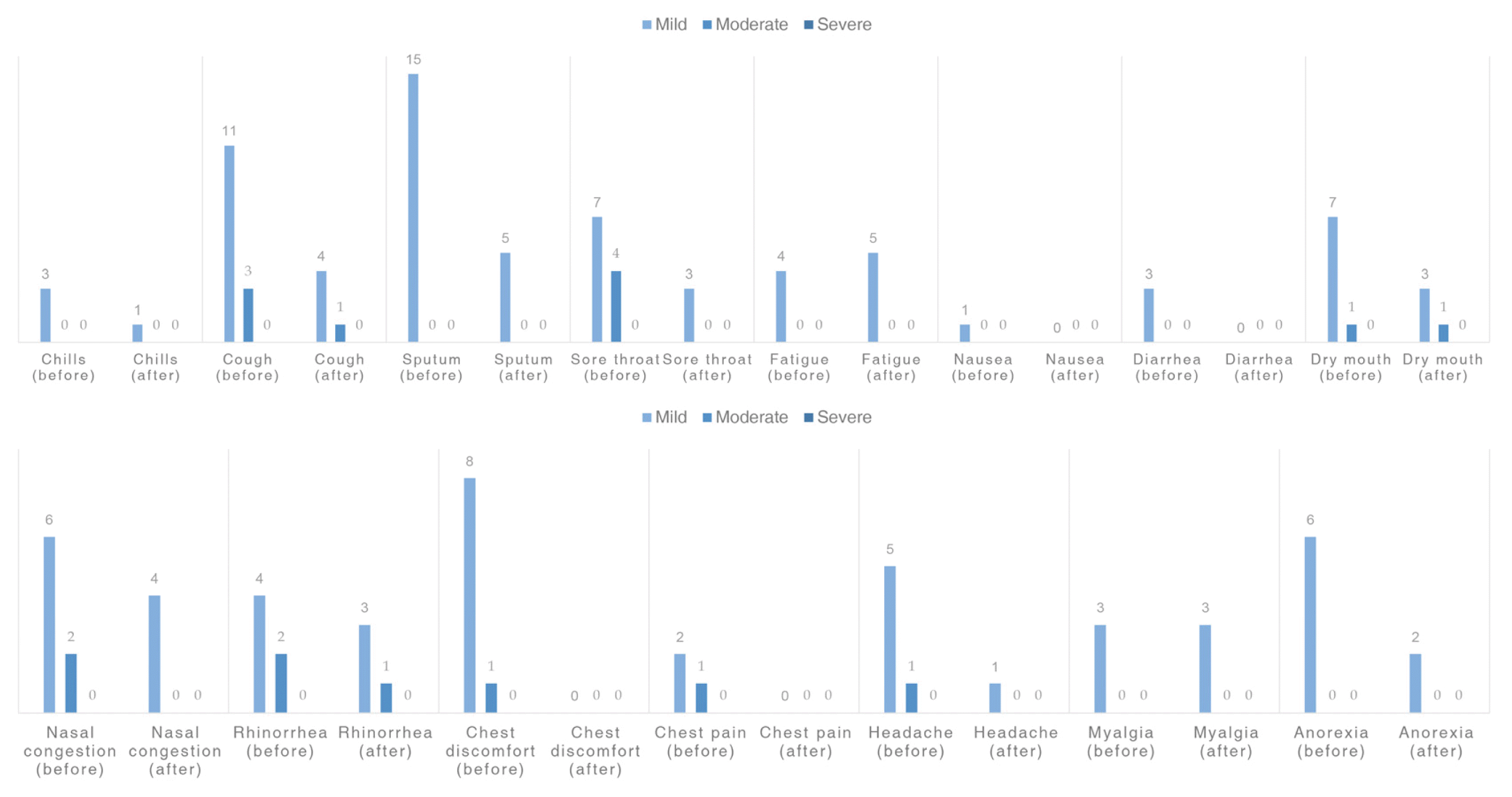
6. Change in the severity of COVID-19-related symptoms after treatment using Korean medicine
The reported patient symptoms during the treatment period were sputum in 15 patients (55.6%), cough in 14 patients (51.9%), sore throat in 11 patients (40.7%), chest discomfort in nine patients (33.3%), dry mouth in eight patients (29.6%), nasal congestion in eight patients (29.6%), rhinorrhea in six patients (22.2%), anorexia in six patients (22.2%), headache or dizziness in six patients (22.2%), fatigue in four patients (14.8%), chills in three patients (11.1%), myalgia in three patients (11.1%), diarrhea in three patients (11.1%), chest pain in three patients (11.1%), and nausea, mild fever, and anosmia in one patient each (3.7%). The changes in symptom severity before and after treatment using Korean medicine are shown in Figure 4.
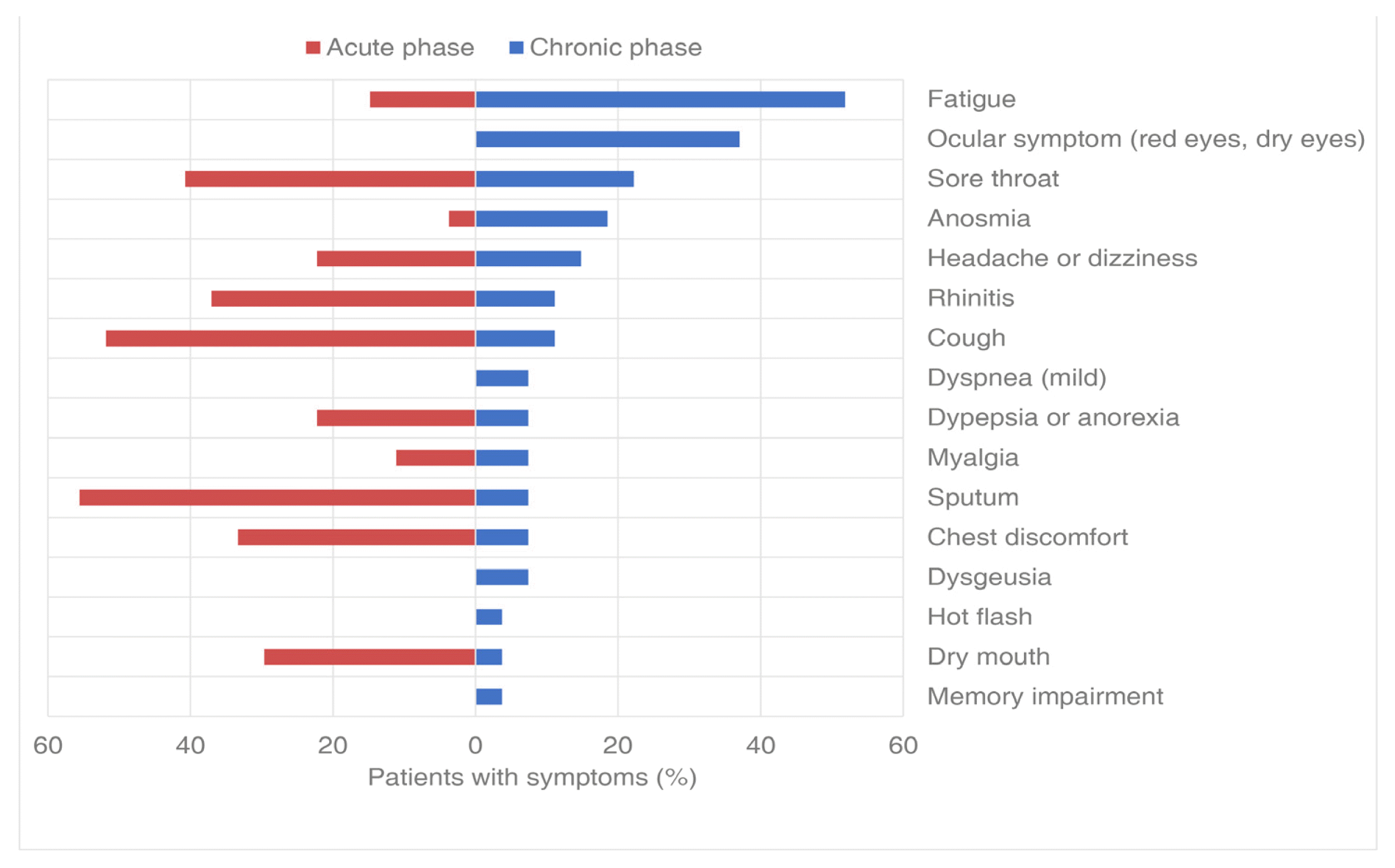
7. Changes in COVID-19-related symptoms during the chronic phase
The chronic phase symptoms observed six months after the end of treatment were investigated through telephone follow up. The chronic phase symptoms included fatigue in 14 patients (51.9%), ocular symptoms (red eyes, dry eyes) in ten patients (37.0%), sore throat in six patients (22.2%), anosmia in five patients (18.5%), headache or dizziness in four patients (14.8%), rhinitis in three patients (11.1%), cough in three patients (11.1%), dyspnea in two patients (7.4%), dyspepsia or anorexia in three patients (11.1%), myalgia in two patients (7.4%), sputum in two patients (7.4%), chest discomfort in two patients (7.4%), dysgeusia in two patients (7.4%), and hot flash, dry mouth, and memory impairment in one patient each (3.7%) (Figure 5).
Discussion
Cheongpebaedok-tang, the main focus of this study, is a prescription for treating the Korean medicine consideration of the pathological mechanism of COVID-19, which appears as cold, dampness, heat, toxin, and deficiency. Cheongpebaedok-tang is a combination n of several Korean medicine prescriptions.9) Animal studies have shown that Cheongpebaedok-tang has antiviral, anti-inflammatory, and metabolic programming effects against COVID-19.10,11) In addition, according to a systematic review and meta-analysis investigating randomized controlled trials, non-randomized trials, cohort studies, and pre-post studies, Cheongpebaedok-tang reduced the nucleic acid conversion time; shortened the hospitalization period; shortened the fever, cough, and chest computed tomography symptom improvement period; reduced the traditional Chinese medicine symptom score; and improved laboratory test biomarkers, including white blood cells, aspartate aminotransferase, and C-reactive protein.12) Based on the above evidence, Cheongpebaedok-tang was adopted as a basic prescription for asymptomatic or mild confirmed cases of COVID-19 in the Korean telemedicine guidelines,3) and was the second most commonly used prescription in the telemedicine center of Korean medicine.13)
According to our results, treatment using Cheongpebaedok-tang reduced the severity of most COVID-19 symptoms. The number of patients complaining of chills, cough, sputum, sore throat, nausea, diarrhea, dry mouth, stuffy nose, rhinorrhea, chest discomfort, chest pain, headache, and anorexia decreased after treatment.
According to a retrospective multicenter cohort study in a comparable timeframe, among 7,057 patients diagnosed with COVID-19 from February 18 to July 10, 2020 in Daegu, 2,162 (30.6%) were asymptomatic, 3,305 (46.8%) had mild disease, and 985 (14.0%) had moderate disease, totaling 6,452 (91.4%). Regarding isolation methods, 202 patients (2.9%) were isolated at home and 2,784 (39.5%) were isolated at a facility. Among the 2,254 patients for whom clinical information could be confirmed, 664 patients (29.5%) had mild symptoms and 985 patients (43.7%) had moderate symptoms, totaling 1,649 patients (73.2%). Cough (38.0%), sputum (29.6%), fever (18.5%), myalgia (17.1%), headache (16.8%), diarrhea (11.4%), sore throat (10.4%), and rhinorrhea (8.4%) were the most common symptoms. For survivors, the mean time from isolation to release was 33 days (interquartile range, 24.0–46.0).14) In a similar period, from January 22, 2020, to May 30, 2020, a survey of 370,000 COVID-19 cases reported to the United States Centers for Disease Control and Prevention revealed that the most common initial symptoms were cough (50.3%), fever (43.1%), myalgia (36.1%), headache (34.4%), shortness of breath (28.5%), and sore throat (20.0%),15) similar to the current study and the retrospective multicenter cohort study. Compared with contemporaneous retrospective studies, patients who were prescribed Cheongpebaedok-tang via telemedicine showed similar results for sputum, cough, sore throat, and rhinorrhea symptoms.
The differences between this study and previous studies are as follows. First, dyspnea was reported less frequently in this study than that reported in previous studies.14,15) Unlike previous studies, only asymptomatic to mild patients were included in the current study; patients with respiratory distress were not included because their severity of illness was evaluated as higher than that of those patients who did not have respiratory distress. Second, dry cough is a common pattern in patients with COVID-19.16) However, our study patients in the acute phase mainly complained of a productive cough with sputum. The different cough patterns may be interpreted as the initial infection being mainly inflammation confined to the nasal passages, throat, and upper respiratory tract in patients with mild symptoms during the acute phase.
Our study revealed both similarities and differences in persistent symptoms from those reported in previous studies. A previous study conducted in Italy, including patients during a similar period from April 21 to May 29, 2020, showed that fatigue was the most common complaint among patients with COVID-19, even in patients in the acute phase.17) Conversely, our study and a study conducted in Daegu14) revealed that fatigue was uncommon in the acute phase. However, fatigue was the only symptom that increased after Korean telemedicine treatment compared to that before treatment, and fatigue emerged as a common symptom in the chronic phase. In addition, ocular symptoms were commonly reported in the present study. Post-COVID-19 fatigue is the most common sequela reported in clinical studies.17–21) Various causes have been suggested, including central factors, such as changes in neurotransmitter levels due to the invasion of the central nervous system by the coronavirus, nerve excitability and inflammation, psychological factors such as fear due to isolation, and peripheral factors, such as skeletal muscle myopathies.22) Symptoms such as conjunctival congestion and dry eye were previously reported in patients with COVID-19,23) and as sequelae, decreased near vision, burning sensation, foreign body sensation, and redness were reported.24) Although the Korean medicine telemedicine guideline suggests convalescent prescriptions for managing sequelae at the last treatment, the current study cannot provide a comprehensive review of the improvement in symptoms after the recovery phase because the treatment was terminated at the same time as the administration of the convalescent prescription. Several studies have purported that herbal medicines are effective in improving symptoms, lung function, and imaging in mild cases and in the management of COVID-19 sequelae.25,26) Given that clinical trials have been registered and are recruiting,27,28) it is expected that additional well-designed large-scale clinical studies will be published in the near future.
The results of this study indicate that telemedicine in Korean medicine is safe. There was no worsening of the severity of COVID-19 during the Korean medicine telemedicine treatment period. A research conducted in March 2020 in Daegu, focusing on asymptomatic and mild COVID-19 cases at community treatment centers, reported that 3% of the participants required hospitalization primarily due to the exacerbation of respiratory symptoms or abnormalities identified in chest radiographs.29) Despite the similarities in recruitment timeframe and symptom severity, none of the patients included in this study were admitted to a hospital due to worsening COVID-19 symptoms. This may be related to the effect of Cheongpebaedok-tang on the decrease in the hospitalization period and symptom relief observed in previous studies.12) Only minor side effects of Cheongpebaedok-tang were investigated in the current study, including heart palpitations, insomnia, dizziness, and diarrhea. Considering that the symptoms improved when the prescription was changed to Cheongpebaedok-tang 2 (Cheongpebaedok-tang without ephedra) or when the number of prescription doses was reduced, the symptoms that improved were consistent with the previously reported side effects of ephedra.30–32) Therefore, Cheongpebaedok-tang appears to be safe, following the recommendation that Cheongpebaedok-tang 2 should be used when side effects are expected.
This study has several limitations. First, as this study took the form of a retrospective case series study through review of medical records, the effect of Cheongpebaedok-tang on the improvement of the patients could only be evaluated using symptoms and not objective indicators such as imaging and blood tests. Fully understanding the effects of Cheongpebaedok -tang in detail will require randomized controlled studies using objective outcome measures such as hospitalization rate, mortality, and recovery period. Further studies involving larger and more diverse patient populations are required. Furthermore, randomized controlled trials are recommended which include studies of both herbal medicine alone and in combination with Western medicine.
The strengths of this study are as follows. First, to our knowledge, this is the first study to evaluate the effectiveness and safety of Cheongpebaedok-tang through telemedicine treatment in Korea. Additionally, this study provided detailed information on patient management by examining the time of self-isolation, confirmation, symptom onset, facility isolation, and release of patients taking herbal medicines, which have not been provided in previously published Korean medicine telemedicine studies. A final strength of this study is that the observation of negative results was likely more accurate because the isolation release criteria in Korea at that time required two negative PCR test results. Notably, unlike previous studies that reported starting treatment from the initial COVID-19 diagnosis, this study showed significant results, even though the treatment was started an average of 19.4 days after diagnosis. This suggests that Cheongpebaedok -tang can be prescribed without being limited for use only as an initial treatment; thus, the scope of application of Cheongpebaedok-tang through telemedicine treatment in clinical settings may be broader than previously thought. The treatment of patients with mild COVID-19 should be safe, have few side effects, and be easy to administer. In particular, telemedicine treatment for patients with mild diseases can protect doctors from exposure, is more convenient and cost-effective, and provides continuous treatment by efficiently allocating limited healthcare resources.33–35) Jang et al. also investigated telemedicine treatment using Korean medicine for COVID-19 and determined that this approach was a satisfactory and convenient treatment for patients, and the second most frequently prescribed herbal medicine was Cheongpebaedok-tang.13) In conclusion, Korean telemedicine management based on the administration of Cheongpebaedok-tang is a potential treatment for patients with mild COVID-19.
In Korean medicine, the visual evaluation of the tongue and sputum is an important component for diagnosing respiratory diseases. The color, shape, and coating of the tongue reflect the condition of the internal organs and the degree and progression of disease.36) Therefore, tongue evaluation represents an important aspect of telemedicine; thus, a method for computerizing and automating tongue diagnosis/evalution is being studied.37,38) The viscosity, quantity, and color of sputum are other important qualities to use for obtaining a diagnosis39); Chinese and Korean COVID-19 medical guidelines also suggest using the appearance of sputum and the condition of the tongue as distinguishing points for symptoms.5,8) Although an evaluation of the tongue and sputum could not be performed owing to the limitations of telephone counseling in the telemedicine center, the accuracy of treatment is expected to increase if automated tongue diagnosis/evaluation and multimedia treatment through sputum pictures are introduced in the future.
Conclusion
This retrospective case series suggested the potential efficacy and safety of Korean telemedicine using Cheongpebaedok-tang for individuals with asymptomatic or mild COVID-19. It indicated relief in major COVID-19 symptoms with manageable, mild side effects, and no exacerbations leading to hospitalization during the treatment period.
Acknowledgments
We are grateful for the support and cooperation of the COVID-19 telemedicine center of Korean medicine and the Association of Korean Medicine.
Notes
Conflict of interest
The authors declare no conflicts of interest for this article.
Funding
This research was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea, No. HI20C1205, for the decision to submit this article for publication.
Ethical statement
This study was approved by the Institutional Review Board (IRB) of Kyung Hee University Korean Medicine Hospital. The IRB approved a waiver of the consent for this study (KOMCIRB 2021-10-007).
Informed consent statement
Patients were not required to give informed consent to the study because the analysis used anonymous clinical data.
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.