Bojungikgi-tang for Anorexia in Lung Cancer Patients with Treated with Chemotherapy: A Single-arm, Open-label, Single-center Trial
Article information
Abstract
Objectives
This study was to evaluate the effectiveness and safety of Bojungikgi-tang for lung cancer patients with anorexia.
Methods
This was a single-arm, open-label, and single-center trial, and suitable participants took Bojungikgi-tang (Buzhongyiqi-tang in Chinese, Hochuekki-to in Japanese) three times a day before or between meals for six weeks (42 days). After registration of clinical trials (visit 2), they visited the hospital every three weeks (visits 3 and 4) and measured or tested the effectiveness or safety evaluation variables to analyze the results. The primary outcome was the anorexia/cachexia subscale (A/CS) of functional assessment of anorexia/cachexia therapy (FAACT) score.
Results
Seventeen lung cancer patients were included in the intention-to-treat (ITT) analysis. Lung cancer patients had higher A/CS of FAACT scores after six weeks of Bojungikgi-tang administration compared to that at the baseline. This was not significant four lung cancer (p=0.1668). In the secondary outcomes, the visual analog scale (VAS) score of anorexia decreased significantly (p=0.0009), and the CD4/CD8 ratio (p=0.0396) and CD4 levels (p=0.0345) significantly increased after six weeks of treatment. No serious adverse events were reported with Bojungikgi-tang in lung cancer patients.
Conclusions
Bojungikgi-tang can be an effective and safe treatment for anorexia in lung cancer patients undergoing chemotherapy.
Introduction
Lung cancer is the second most common cancer in the world, with more than 2.2 million new lung cancer patients in 20201), and approximately 85% of lung cancer is non-small cell lung cancer (NSCLC)2). The treatments of NSCLCs include surgical resection, chemotherapy, and radiation therapy, and the treatment method is determined by considering the stage and metastasis of cancer. Surgery is the best treatment for stage I or II NSCLC patients, and chemotherapy is recommended for stage IIIB or IV patients. Combining cisplatin with docetaxel, etoposide, gemcitabine, or vinorelbine is recommended as adjuvant therapy for locally advanced disease. Also, specific targeted therapy or immunotherapy may be performed in patients suitable for molecular and biomarker analysis3). Small cell lung cancer (SCLC), which accounts for approximately 15–20% of lung cancer, reacts relatively well to chemotherapy, but it has a high recurrence rate and a low survival rate4). In patients with SCLC, chemotherapy is the basic treatment, etoposide plus cisplatin is the first recommendation in the limited-stage, and chemotherapy plus immunotherapy are the first recommendation in the extensive stage. Combination therapy using cisplatin is also used for SCLC, and carboplatin has been commonly used due to its low nephrotoxicity and neurotoxicity while having the same effect as cisplatin5).
Cisplatin, the primary platinum-based agent of chemotherapy, causes nausea and vomiting as a side effect. Furthermore, cisplatin releases large amounts of serotonin binding to 5-HT receptors in vivo, resulting in anorexia by regulating the secretion of acyl ghrelin. Even if chemotherapy-induced nausea and vomiting are alleviated by antiemetic medication, the chemotherapy-induced anorexia persists, preventing patients from maintaining adequate nutrition and reducing their quality of life, making it difficult to complete chemotherapy6,7).
Currently, medication for cancer-related anorexia can be classified into appetite promoters, anti-inflammatory drugs, and metabolic drugs8). An appetite promoter, megestrol acetate is mainly used for cancer-related anorexia. However, it can cause adverse effects such as thromboembolism, sexual dysfunction, and vaginal bleeding9,10). Cyproheptadine can cause sedation and drowsiness due to its antihistamine effect. However, insufficient clinical data supporting anti-inflammatory drugs has been reported, and some types may have serious adverse effects. Metabolic drugs have not been used due to severe concerns over the possibility of tumor growth and a decrease in body defense mechanisms8).
Lung cancer patients treated with chemotherapy may be interested in safer alternative medicine to treat chemotherapy-induced anorexia, and herbal medicine treatment can be a safe alternative treatment. Bojungikgi-tang (Buzhongyiqi-tang in Chinese, Hochuekki-to in Japanese) is a widely prescribed in East Asian countries such as Korea, Japan, China, and Taiwan, and is mainly used to treat weakness and anorexia, and improve digestive function and immunity11). According to Korea's Ministry of Food and Drug Safety, Bojungikgi-tang is used for the following symptoms: general weakness, fatigue, weakness after disease, anorexia, and sweating while sleeping at night. Studies reported that Bojungikgi-tang has fewer adverse effects while improving the appetite and the quality of life of lung cancer patients treated with cisplatin chemotherapy12). As for the pharmacological mechanism of Bojungikgi-tang for the anorexia, there is a study on the effects of the constituent herbs, Aurantii nobilis Pericarpium and Atractylodis Lanceae Rhizoma. Hesperidin, an active substance of Aurantii nobilis Pericarpium promotes the secretion of ghrelin by antagonizing 5-HT2cR, and atractyrodin, an active substance of Atractylodis Lanceae Rhizoma, acts on the end of the vagus nerve to strengthen the action of ghrelin13).
Bojungikgi-tang is likely to be considered a safe treatment for cancer patients to treat chemotherapy-induced anorexia. Therefore, this study aimed to explore the effectiveness and safety of Bojungikgi-tang for anorexia in lung cancer patients treated with chemotherapy.
Methods
1. Study Design and Setting
This single-arm, open-label, and single-center trial was conducted at the Pusan National University Korean Medicine Hospital in Pusan, Korea, from January 27, 2020 to December 31, 2021. Bojungikgi-tang was administered three times a day for 42 days (6 weeks) before or during the meals to investigate its effect on chemotherapy-induced anorexia in lung cancer patients. The patients visited the hospital every three weeks after registration and underwent tests for effectiveness and safety evaluation.
2. Participants
An advertisement was posted at the respiratory clinic of this hospital to recruit patients. Physicians interviewed the participants to confirm their clinical condition and determine whether they met the study criteria. The data required for the study were collected by the clinical research coordinator. The participants provided informed consent for voluntary participation in the trial and observing the precautions.
1.2 Inclusion Criteria
Adults aged ≥ 19 years
Participants with histologically or cytologically confirmed lung cancer (NSCLC [Ib~IV] or SCLC [extensive stage])
Participants who had received chemotherapy containing a platinum-based anticancer drug (cisplatin or carboplatin) at least one time at the screening visit and scheduled for chemotherapy at least two times after the screening visit
Participants able to ingest medicine orally
Eastern cooperative oncology group (ECOG) performance status score ≤ 2
Participants who were suffering from anorexia after chemotherapy, and score on the VAS for anorexia ≥ 40/100 mm (100 mm is the worst anorexia score)
Participants who were not taking megestrol acetate for stimulating appetite
After hearing and fully understanding the detailed description of the clinical trial, individuals who provided written informed consent for voluntary participation and observing the precautions
Participants who could fully communicate with their doctor about their symptoms or quality of life and who could fill out questionnaires
Participants who could follow-up during the clinical trial
Participants with a life expectancy of three months or more
1.2 Exclusion Criteria
Individuals who had hemoptysis (> 2.5 mL/day) within 2 weeks at the screening visit
Individuals who had symptomatic and uncontrolled brain or central nervous system metastasis, have other malignancies or with a history of other malignancies within five years from the screening visit (Except cured basal or squamous cell skin cancer, cervical epithelial cancer, thyroid cancer, prostate cancer or breast cancer)
Total bilirubin level higher than 2.0 mg/dL
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels higher than 2.5 times the upper limit for normal
Creatinine level higher than 1.5 times the upper limit for normal
Individuals who had uncontrolled hypertension (Diastolic blood pressure > 100 mmHg or systolic blood pressure > 160 mmHg), diabetes, active infection, or heart diseases such as symptomatic congestive heart failure or unstable angina
Individuals who took other herbal medicine within two weeks of the screening visit
Individuals with uncontrolled pleural effusion, ascites, pericardial effusion
Individuals who had a major psychotic disorder diagnosed by Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) that is taking or should be taking psychiatry medications (except insomnia)
Individuals who had alcoholism or drug dependence
Individuals with a history of serious drug allergies or who have hypersensitivity to the investigational product (the main ingredient and its components)
Individuals who had genetic problems such as galactose intolerance, lapp lactase deficiency, or glucose-galactose malabsorption
Individuals who were pregnant, breastfeeding, planning to become pregnant or women of childbearing potential who disagreed with the appropriate method of contraception
Individuals who had participated in other clinical trials within six months (180 days before screening visit) or planned to participate in other clinical trials during the trial
Individuals who had a medical condition that would have likely affected the results or who were deemed to be inappropriate to participate by an investigator
Individuals who required or received parenteral or tube nutrition
3. Sample Size
For the sample size calculation, a previous study in which Sipkeondaebo-tang was administered for four weeks to improve the anorexia of cancer patients was referenced14). The mean and standard deviation for the difference before and after of the A/CS scale of FAACT were 4.63 and 4.50, respectively. The value calculated by the G-power program was 13 (A two-sided paired t-test, a error : 0.050, b error : 0.10). Since this study involved the administration of drugs for six weeks, the target number of participants was calculated considering a dropout rate of 35%. Therefore, the sample size was calculated as 20.
4. Preparation of Bojungikgi-tang
The Bojungikgi-tang used in this study was provided by pharmaceutical company Hanpoong Pharm & Foods Co (Wanju-gun, Korea) under Good Manufacturing Practices. The participants were instructed to take one pack (75 mL) orally before or during their meals three times a day.
The Bojungikgi-tang reference standards used in chemical profiling were all over 98.0% and were purchased from standard manufacturers — Biopurify Phytochemicals (Chengdu, China), ChemFaces Biochemical Co. (Wuhan, China), and Fujifilm Wako Pure Chemical Co. (Osaka, Japan). Water, acetonitrile, and acetic acid were high-performance liquid chromatography (HPLC) grade and purchased from J.T. Baker (Phillipsburg, NJ, USA) and Merck (Darmstadt, Germany), respectively. Chemical profile analysis of Bojungikgi-tang drug using HPLC was conducted according to previously reported research method 15). Briefly, chemical profiling was performed using a Gemini C18 analytical column (250 × 4.6 mm, 5 μm, Phenomenx, Torrance, CA, USA) and a water–acetonitrile (both contain 1.0% acetic acid) mobile phase on a Shimadzu Prominence LC–20A series system (Kyoko, Japan).
5. Outcomes
The primary outcome was the functional assessment of anorexia/cachexia therapy (FAACT) anorexia/cachexia subscale (A/CS), on visits 4 (6 weeks after baseline). The secondary outcomes were FACT-G, FACT-L, visual analogue scale (VAS) of anorexia, weight, body mass index (BMI), skeletal muscle mass and CD4, CD8 as wells as CD4/CD8 ratio in blood.
6. Safety
Participants who took Bojungikgi-tang more than once after registration in this clinical trial were subject to safety evaluation. Safety evaluation includes adverse reactions, clinical laboratory tests, vital signs, and physical examinations. Researchers were instructed to explain all adverse reactions that may occur after drug administration to participants or guardians and report all symptoms after treatment. An adverse reaction is analyzed by symptom, and the frequency is recorded in the adverse event report.
7. Statistical Analysis
The main analysis is a full analysis set (FAS) analysis according to the intention-to-treat (ITT) principle, and the secondary analysis is per protocol (PP) analysis for the effectiveness evaluation. The last observation carried forward (LOCF) method was used when the missing value was found in the ITT analysis. This is to replace the missing value in visit 4 (6 weeks after baseline) with the matter in visit 2 (baseline) or 3 (3 weeks after baseline)). The statistical significance criterion for primary and secondary outcome is a significance level of 5%. For continuous data, a paired t-test is performed for normal distribution and a Wilcoxon signed rank test for not normal distribution. All analyses were performed using the SPSS 23 for Windows (SPSS inc., Chicago, IL, USA) program.
The adverse events were analyzed for each symptom and expressed as a frequency for the safety analysis.
Results
1. Baseline and Disease Characteristics of Patients
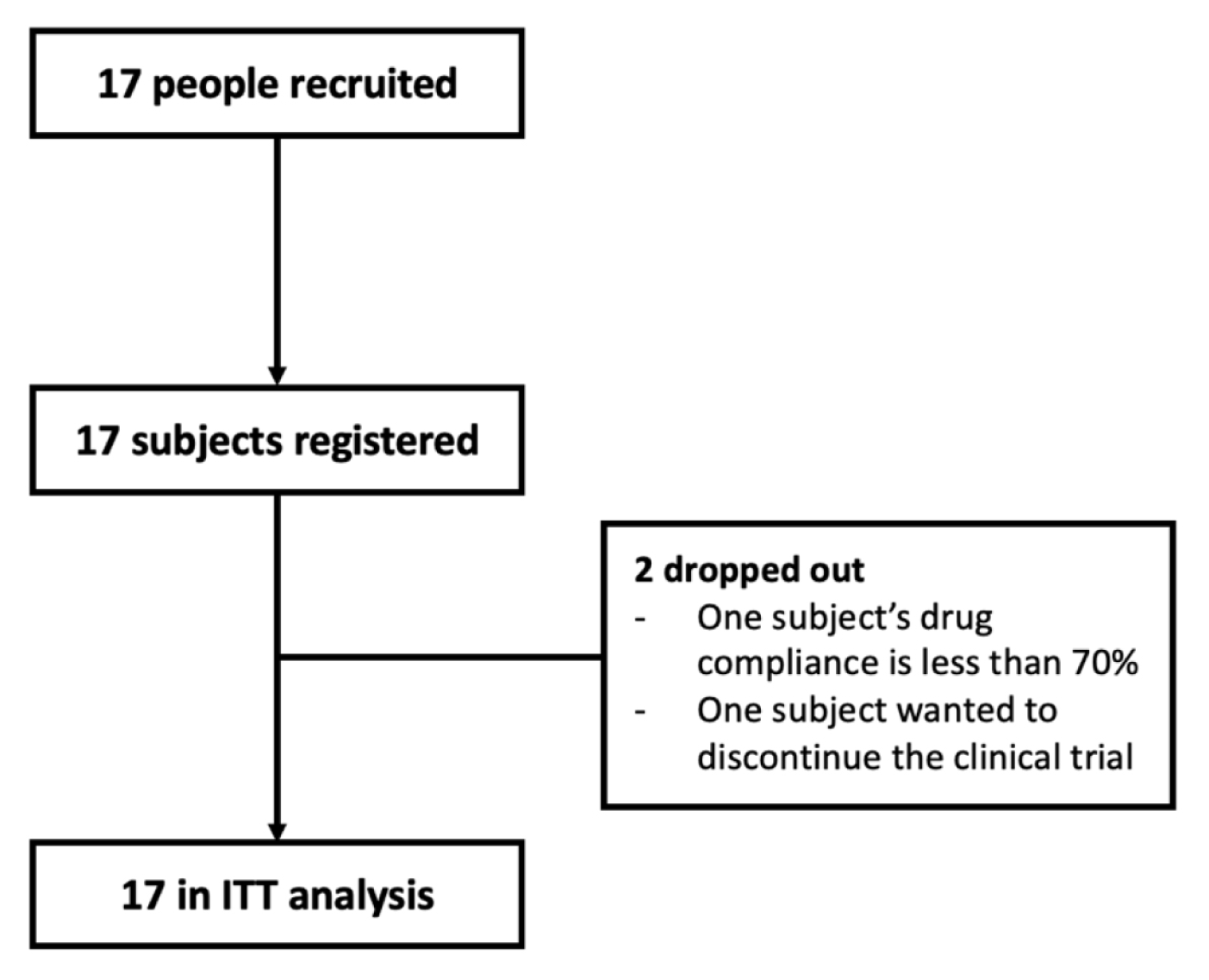
A total of 17 participants participated in this study, but two did not complete (Figure 1). The characteristics of the participants’ baseline and disease are shown in Table 1.
2. Primary Outcome
The primary outcome was the A/CS of FAACT, and the mean scores on visits 2 (baseline) and 4 (6 weeks after baseline) were 30.4±5.5 and 32.8±7.6, respectively. The mean difference was 2.4±6.7 with no significant difference between the values at the baseline and at visit 4 (Table 2).
3. Secondary Outcomes
In FACT-G and FACT-L, no significant differences were observed between the values at the baseline and at visit 3 (3 weeks after baseline) or 4 (6 weeks after baseline). In the VAS of anorexia, no significant difference was identified between the values at the baseline and visit 3; however, the VAS score for anorexia decreased significantly at visit 4 compared with that at the baseline (p=0.0009). Comparing the values at visits 3 and 4 with those at the baseline, there were no significant differences in weight, BMI, and skeletal muscle mass (Table 3).
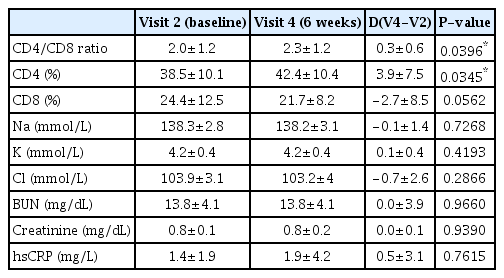
At visit 4, CD4/CD8 and CD4 levels increased significantly compared with their baseline levels (p=0.0396 and 0.0345, respectively), while there was no significant difference in the CD8 levels (p=0.0562). There were no significant differences between the baseline and visit 4 in terms of metabolic test results and hsCRP levels (Table 4).
4. Safety
There were no serious adverse events, and all those reported during the study were alleviated. During the study period, the symptoms of adverse events in the participants included vomiting, nausea, dizziness, sweating, general weakness, heartburn, high blood pressure, headache, constipation, and bloating (Table 5).
5. HPLC Chemical Profiling of Bojungikgi-tang Drug
For simultaneous quantitative analysis of the four components (liquiritin, nodakenin, hesperidin, and glycyrrhizin) from the Bojungikgi-tang drug using HPLC coupled with a photo-diode array detector, a previously reported regression equation was used15). Glycyrrhizin at 254 nm, liquiritin and hesperidin at 280 nm, and nodaknin at 335 nm were quantified, respectively (Figure 2). As a result, the four components (liquiritn, nodakenin, hesperidin, and glycyrrhizin) was detected at 10.79±0.15, 13.30±0.17, 58.82±0.89, and 28.91±0.68 g/mL, respectively.
Discussion
Of the total 17 patients registered in this study, 16 were NSCLC patients, excluding one SCLC patient. The stage was based on the final diagnoses at the clinical or postoperative stage; stage IV patients were the most common, with eight (47.06%). Eleven patients (64.71%) underwent chemotherapy after surgery, and all patients underwent chemotherapy with platinum-based agents. Approximately 70% of lung cancer patients are diagnosed with advanced stage, and 40% of patients are in stage IV, so chemotherapy is inevitable for advanced lung cancer patients16).
Platinum anticancer drugs have serious side effects. Cisplatin has nephrotoxicity, carboplatin has myelosuppression, and oxaliplatin has neurotoxicity. Also, there is a possibility of side effects such as hypersensitivity, cytopenia, hepatotoxicity, nausea, vomiting, diarrhea, stomatitis, and anorexia. Approximately 67% of lung cancer patients with chemotherapy suffer from anorexia, especially those who received cisplatin chemotherapy17,18). The survival rate of lung cancer patients has been increasing due to chemotherapy, but the quality of life is not. Therefore, the need for palliative treatment for cancer symptoms and side effects of chemotherapy is increasing19,20). Consequently, it is necessary to treat cancer patients suffering from chemotherapy-induced anorexia, and we used Bojungikgi-tang as a research drug.
Bojungikgi-tang was made around the 13th century and has been used frequently in East Asian countries until now. It treats general weakness, fatigue, anorexia, indigestion, and weakened immunity21). In addition, there are reports that Bojungikgi-tang improves the toxic side effects of chemotherapy. This drug is used as an adjuvant to improve the quality of life of cancer patients receiving chemotherapy22). In a study in Taiwan, lung cancer patients who received herbal medicine had a lower mortality hazard ratio than those who did not, and the most used herbal medicine was reported as Bojungikgi-tang23). Bojungikgi-tang is also related to the resistance of patients receiving cisplatin treatment. In the in vitro experiment, it was reported that Bojungikgi-tang inhibits the growth of cisplatin-resistant lung cancer cells24). Therefore, in this study, various outcomes were set up to find out the effectiveness and safety of Bojungikgi-tang on chemotherapy-induced anorexia in lung cancer patients.
FAACT is a reliable questionnaire that evaluates the quality of life of cancer patients with anorexia/cachexia25). There is no significant difference between before and after of A/CS of FAACT, the primary outcome of this study. However, referring to a study analyzing that the cut-off value of A/CS of FAACT score is less than 32 in patients with Non-small cell lung cancer26), the mean score of baseline in this study is 30.4 and the mean score after six weeks of administration is 32.8, so anorexia is improved above the cut-off.
FACT-G is a questionnaire that has been used in several studies to evaluate the quality of life of lung cancer patients. It is divided into four parts (physical, social/family, emotional, and functional) and consists of 27 questions. In addition, FACT-L consists of nine additional questions that evaluate lung cancer-specific symptoms27,28). In one study 29) comparing FACT-L between a group with traditional Chinese medicine (TCM) treatment and chemotherapy and a group with chemotherapy only, the group with TCM treatment had significantly higher scores than those of the chemotherapy group. Comparing the post anticancer score of the TCM group with that at the baseline, no significant difference was observed. However, the scores were gradually decreased in the subscales of physical well-being and lung cancer symptom, but the decrease was less than in the chemotherapy alone group. In our study, FACT-G and FACT-L also had no significant changes before and after the medication. The results of the two questionnaires gradually improved after six weeks compared to those at the baseline, but could not be compared with a control group receiving only chemotherapy. Nevertheless, there was a significant decrease between baseline and six weeks in VAS of anorexia. This means that the anorexia that patients feel subjectively has significantly improved after taking Bojungikgi-tang.
T lymphocytes play a role in the innate anticancer immune response. In several types of cancers, the immune cells associated with clinical efficacy and survival are tumor-infiltrating CD4+ and CD8+ T cells. CD4+ T cells help the killing activity of cytotoxic CD8+ T lymphocytes and other hematopoietic cells such as NK cells, DCs, and macrophages in the immune system. CD4+ T cells are activated after receiving specific antigen peptides through the major histocompatibility complex (MHC) class II molecule of antigen presenting cells (APCs). Activated CD4+ T cells cause rapid proliferation and secretion of cytokines that regulate immune responses. The T cell receptor (TCR) of CD8+ T lymphocytes recognizes the specific antigen peptide presented by human leukocyte antigen class I (HLA-I)/beta-2-microglobulin (β2m) complexes on the surface of the target cell. Activated CD8+ T lymphocytes play an essential role in anticancer immunity by killing tumor cells directly30,31). In addition, Certain types of blood CD4+ T cells are also known as good prognostic factors in lung cancer patients32).
When the immune function of cancer patients is impaired, CD4 cells and CD4/CD8 decrease and CD8 cells increase33). A low CD4/CD8 ratio means that the cell-mediated immune function of cancer patients decreased31). Studies show an increased CD4 and CD4/CD8 ratio by administering herbal medicines to lung cancer patients. In a study by Yang et al., patients with stage III and IV lung cancer using both herbal medicine and chemotherapy showed significantly higher CD4 and CD4/CD8 than those who received chemotherapy only34). In another study by Li et al., Fu-Zheng-Qu-Xie (扶正祛邪) was administered to patients with early-stage lung cancer and after administering FZQX, the recurrence rate of 3-year post-operation was significantly reduced, and the quality of life was improved. Moreover, CD4/CD8 was increased in peripheral blood tests 35). In addition, one study found that Bojungikgi-tang can reduce the upregulation of CD8 T cells induced by chemotherapy36).
In our study, CD4 and CD4/CD8 ratio in the blood were significantly increased in patients who received both chemotherapy and Bojungikgi-tang. Whether this positively affects the patient's condition will need to be revealed through a further study of various subtype analyses of CD4 and CD8 cells.
In one randomized controlled trial (RCT) in lung cancer patients, the group that combined herbal medicine and adjuvant chemotherapy had less decrease in hemoglobin and less increase in total bilirubin compared to that in the control group. In that study, adjuvant therapy, including platinum-based anticancer agents, was used as control, and it is mentioned that the abnormal level was normalized when the anticancer cycle was completed37). In our study, lung cancer patients receiving chemotherapy including platinum-based anticancer agents, showed a decrease in hemoglobin and an increase in total bilirubin after administration compared to that before the administration of Bojungikgi-tang. This might have been due to hematological toxicity and hepatotoxicity, among various side effects that may appear in platinum-based anticancer agents18). In other safety tests, there was no significant change before and after the administration of Bojungikgi-tang. All seven patients who reported adverse events showed mild symptoms, and all of them were alleviated. A study taking herbal medicine as a supplementary treatment for lung cancer patients under chemotherapy reported constipation and mild blood pressure increase38).
Our study had the following limitations. First, it was a single-group study, and included no control group, and the number of participants was not large, making it difficult to generalize the results of this study. A well-designed and large-scale comparative study is needed in the future. Second, since it was targeted at patients undergoing chemotherapy, the effect of chemotherapy on patients cannot be excluded, and it is unclear. However, this study is meaningful as no serious side effects were identified while showing effective results on appetite and improved immunity by administering Bojungikgi-tang to lung cancer patients with chemotherapy-induced anorexia.
Collectively, Bojungikgi-tang could be an effective and safe treatment without serious adverse events for anorexia in lung cancer patients undergoing chemotherapy. To confirm this, future well-designed trial for anorexia containing immune function monitoring should be needed.
Data availability
Because of ethical concerns, supporting data cannot be made openly available.
Acknowledgment & Funding
This research was supported by grants from the Korea Institute of Oriental Medicine (KIOM) (grant numbers: KSN1812240, KSN2013310, and KSN2021310).
Notes
Author contributions
IH Choi and SH Yoon drafted the manuscript. SY Kim and YE Choi interpreted data. BK Kang conducted statistical analysis of clinical data. CS Seo analyzed the HPLC data of Bojungikgi-tang. JY Choi and HK Shin designed the study and supervised the research.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical statement
This research was reviewed and approved by the institutional review board (IRB) of the Pusan National University Korean Medical Hospital (registration number PNUKHIRB-2019011). Informed consent was obtained from all participants.
This research was supported by grants from the Korea Institute of Oriental Medicine (KIOM) (grant numbers: KSN1812240, KSN2013310, and KSN2021310).