Fetal safety of medicinal herbs and food ingredients during pregnancy: Recommendations from traditional Korean medicine based on expert opinions
Article information
Abstract
Objectives
This study aimed to establish and provide reliable information for general public, based on expert consensus, on the risks of misuse of medicinal herbs for food and pure food ingredients for the fetus during pregnancy.
Methods
A panelist of seven traditional Korean medicine (TKM) gynecologists responded to a questionnaire summarizing the fetal safety literature for twenty-five medicinal herbs for food and pure food ingredients derived from medicated diet (藥膳, Yaksun) recipes during three online Delphi rounds anonymously.
Results
Ginkgonis Semen (Ginkgo nut), Illici Veri Fructus (Star anise), lavender, bitter gourd, and parsley were agreed at the level 1 of “Do not consume”. These five ingredients were recognized as having significant risks both in the literature evidence and in expert opinion. Rosemary, Citri Unshius Pericarpium, Discoreae Rhizoma, lemongrass, Schisandrae Fructus, Cassiae Semen, Foeniculi Fructus, Mori Fructus, Cinnamomi Cortex, and Astragali Radix were agreed at the level 2 of “consultation with TKM practitioner is required”.
Conclusion
Based on the consensus of a seven-member expert panel of TKM gynecologists, consumption of Ginkgonis Semen (Ginkgo nut), Illici Veri Fructus (Star anise), lavender, bitter gourd, and parsley should be avoided by pregnant women. For Rosemary, Citri Unshius Pericarpium, Discoreae Rhizoma, lemongrass, Schisandrae Fructus, Cassiae Semen, Foeniculi Fructus, Mori Fructus, Cinnamomi Cortex, and Astragali Radix, the level 2 advisory may be recommended to use with caution and to consult a TKM practitioner for advice on consumption, dose, and duration.
Introduction
The number of medicinal herbs for food use, which can be institutionally marketed as both agricultural products and specialized medicinal herbs, is relatively high in Korea compared to China, Japan, and Taiwan and some of these medicinal herbs for food use in Korea are not allowed to be used as medicinal purpose in Japan. Experts in Korea have been calling for complementing the system to prevent these potential public risks from misuse1). On the other hand, the risks of misuse of medicinal herbs are not well known to the general public, who are often unaware of the dangers posed by the easy availability of medicinal herbs in the marketplace, both online and offline2). A study using Naver DataLab service to analyze the search trends of health food consumers’ online purchases of medicinal herbs reported that many medicinal herbs are purchased to drink boiled extract water or to use as an ingredient in ordinary food, and that the search volume increased with the trend cycle of a particular medicinal herb3). On the other hand, demands for dietary supplements including medicinal herbs in Korea is growing steadily every year4). A study that examined the food ingredients used in Korean medicated diet restaurants reported that ninety-six medicinal herbs for food use were used, including some that could be recognized as specialized medicinal herbs such as Paeoniae Radix, Atractylodis Rhizoma Alba, Cervi Parvum Cornu, Acori Graminei Rhizoma, Leonuri Herba, and Araliae Continentalis Radix5). This is considered to be owing to the fact that some people in Korea still have a false perception that naturally grown and harvested agricultural products, whether they are used as medicinal herbs or not, are safe enough when even taken for a long period6).
East Asian countries have a long history of public use of medicinal herbs in their daily life, and the line between medicinal herbs and foods has always been blurred from the beginning. Therefore, the research of medicinal herbs for food use has not solely been limited to therapeutic purpose. In Korea, dietary therapy (食治) using medicinal herbs for food use is known to begin to be practiced professionally in the Chosun Dynasty, and the ingredients including medicinal herbs used in medicated diets are recorded in farming books related to cultivation, gathering, and production, geographical map books related to major production areas, and food recipe books7). According to a study that systematically reviewed the trends of medicated diets research in Korea, it was found that more research was conducted on food science value and productivity than on the efficacy of medicinal herbs, which was conducted by experts in traditional Korean medicine (TKM). In addition, the researchers’ specialties were mainly focused on food nutrition and food science majors8).
Moreover, in pregnant women who are carrying a fetus that is vulnerable to the potential hazards posed by these environments, the potential for adverse, even minor, effects on the fetus cannot be ruled out enough. However, it is not easy for pregnant women in Korea to access reliable safety information on medicinal herbs for food use. Therefore, we anticipated that a large number of pregnant women would likely make their consumption decisions based on internet media such as searching portal websites, online communities, or unsubstantiated information from acquaintances9). The fetal safety of herbs used abroad has been extensively reported at the literature review level, however the medicinal herbs for foods and pure food ingredients used in Korea are different from those used abroad. Therefore, it is necessary to set up a list of medicinal herbs for food and pure food ingredients and conduct research according to domestic environments10,11,12).
In this study, we created a Delphi questionnaire based on literature including previously reported clinical, animal experimental, and literature review studies, and conducted an expert consensus online Delphi study with seven TKM gynecologists working in pregnancy care practice setting. On the one hand, we aimed to secure the safety of unborn children who are vulnerable to this risk by preventing the potential risk of misuse of medicinal herbs for food. On the other hand, we aimed to help general public by providing relatively objective information on misconceptions about food ingredients that are often dismissed without any evidence. In the future, we would like to disclose this consensus information to the general public in the form of individual safety information for each herb or ingredient.
Methods
1. Collecting literatures and creating questionnaire form
We collected medicated diet and herbal tea recipes published on the popular internet web portals including Naver (http://www.naver.com/), Baidu (http://www.baidu.com/), Google (http://www.google.com/) and YouTube (http://www.youtube.com) to extract the list of medicinal herbs for food and pure food ingredients, and collected and analyzed the fetal safety literature on them. Due to the nature of medicated diets, we determined that recipes from ancient sources are not currently applicable in practice. Therefore, we used common portal websites for collecting recipes that seemed to be available today. Search terms such as “食疗” (diet therapy), “药膳” (medicated diet), and “药茶” (medicated tea) were used and recipes were mainly collected from videos on the medicated diet YouTube channel 侯老师说食疗, posts on the websites such as 解疑经验百科 (https://mip.jiankangti.com/), 360doc个人图书馆 (http://www.360doc.com/), and 百度百科 (https://baike.baidu.com/). The scope of the safety assessment was limited to the safety of fetal developmental abnormalities and miscarriage during pregnancy, however did not include maternal safety during pregnancy, safety during labor, or fetal safety during breastfeeding period. Among the candidate ingredients, a list of medicinal herbs for food and pure food ingredients deemed to have a high risk to the fetus was derived, and medicinal herbs that are not medicinal herbs also for food use based on the Food Standards and Standards Act in Korea was excluded. Fetal safety literatures until December 29th, 2021 were collected from Google Scholar (http://scholar.google.com/) and PubMed (https://pubmed.ncbi.nlm.nih.gov/) databases using the following English search terms: each herbs and food name with scientific name, “pregnancy”, “pregnant”, “fetus”, “fetal”, “safety”, and “toxicity”. Of the literature collected and reviewed, the final 86 articles were included as references in the questionnaire. After prior consultation with experts, including five TKM gynecologists, four levels of caution for pregnant women’s consumption of medicinal herbs for foods and pure food ingredients was derived, and these four levels were presented as options in a Delphi consensus questionnaire summarizing the fetal safety literature for each ingredient. In presenting the safety summaries of the literature papers by ingredients, we categorized ingredients by human clinical, animal experimental, and literature review studies.
2. Recruiting Delphi expert panels
We officially requested recommendations to the Society of Korean Medicine Obstetrics and Gynecology and received a list of twenty-one TKM gynecologists in pregnancy care setting including information of name, phone number, email address, gender, age, practice area, and affiliation. A final seven-member expert panel was selected based on considering diversity of practice setting (University hospital, general hospital, and clinic) and geography, professional recognition, and age distribution. The selected expert panels were individually informed of the purpose and methodology of this Delphi study and asked to agree to participate from a moderator. The Delphi consensus questionnaire was emailed to each panel and they were instructed by the moderator to select a rating level of caution for each ingredient, along with the reasons for their choices. The study protocol including methodology for collecting expert information and disclosing consensus results was approved by the Institutional Review Board of the Korea Institute of Oriental Medicine (I-2210/010-002). The Institutional Review Board were responsible for supervising all aspects of the study.
3. Delphi process and consensus criteria
The Delphi research process was conducted exclusively online in three rounds from March 2nd to April 19th, 2023, and any disagreements in each Delphi round were resolved in the next round of consensus. The criteria for determining agreement and disagreement, which were established upon providing the questionnaire, were as follows, and were presented in the instructions for the initial distribution of the questionnaire: i) For each ingredient, consensus or agreement was defined as an intake caution rating selected by at least four of seven panels and differing by at least two points from the next highest frequency rating; ii) For each ingredient, disagreement was defined as the caution rating with the highest frequency less than 4 or differing between the most and next frequent ratings less than or equal to one. If the same item was disagreed in a similar distribution of response during two rounds of Delphi, the following revised criteria for third round was defined and distributed to the panels. If the third round of consensus resulted in a disagreement based on the initial judgement criteria, the moderator would simply determine the most frequent caution rating as agreed, however if two caution ratings were selected with equal frequency, a stricter level of rating was selected as consensual.
The results of each Delphi round were shared anonymously with all panelists, including the reasons for their choices prior to the next Delphi round, and the consensus process was repeated on the same questionnaire with only the item that was not agreed upon. All expert opinions remained anonymous from the beginning of the Delphi consensus to the end of the study.
Results
1. Deriving ingestion caution levels
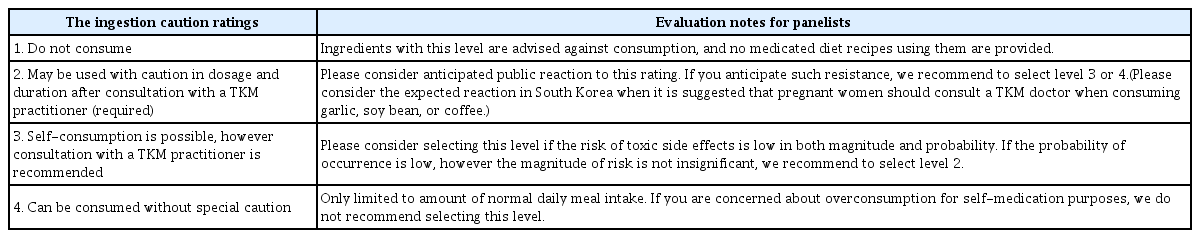
The ingestion caution levels were derived as follows: 1. Do not consume; 2. May be used with caution in dosage and duration after consultation with a TKM practitioner (required); 3. Self-consumption is possible, however consultation with a TKM practitioner is recommended; 4. Can be consumed without special caution. The ingestion caution levels, along with the evaluation notes provided to the panelists, are presented in Table 1.
2. Ingredients included in the Delphi questionnaire
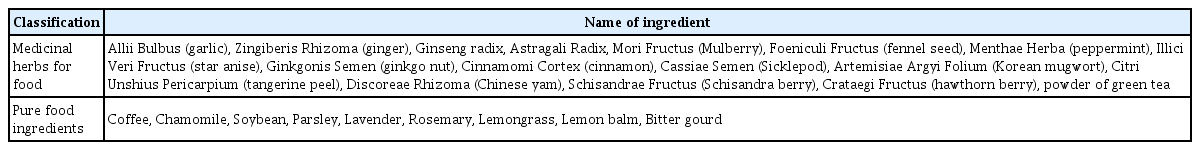
In total, the literatures for twenty-five medicinal herbs for food and pure food ingredients were summarized and references were provided to the panelists along with the type of evidence. Fourteen ingredients had clinical research evidences, eight ingredients had no clinical research evidences however animal research evidences, and three ingredients had only literature review evidences in the Delphi questionnaire. The classification and list of medicinal herbs for food and pure food ingredients are presented in Table 213). Clinical research evidence included three Cochrane systematic reviews, seven randomized controlled trials, and four prospective cohort studies, with the most clinically studied ingredients being coffee/green tea (caffeine), Allii Bulbus (garlic), Zingiberis Rhizoma (ginger), Ginseng Radix, and chamomile.
3. Delphi panelists and process
The panelists consisted of five professors from TKM university hospital, one specialist from a TKM general hospital, and one specialist from a TKM clinic. The expert panelists’ practice locations were relatively evenly distributed across the country, including Seoul, Gyeonggi-do, Chungcheongbuk-do, Daejeon, Jeollanam-do, Gyeongsangbuk-do, and the average age of the panelists was 48.7 years, with a 5:2 male-to-female ratio. The expert Delphi consensus process took place online in three rounds from March 2nd to April 19th, 2023.
4. Expert Delphi consensus results
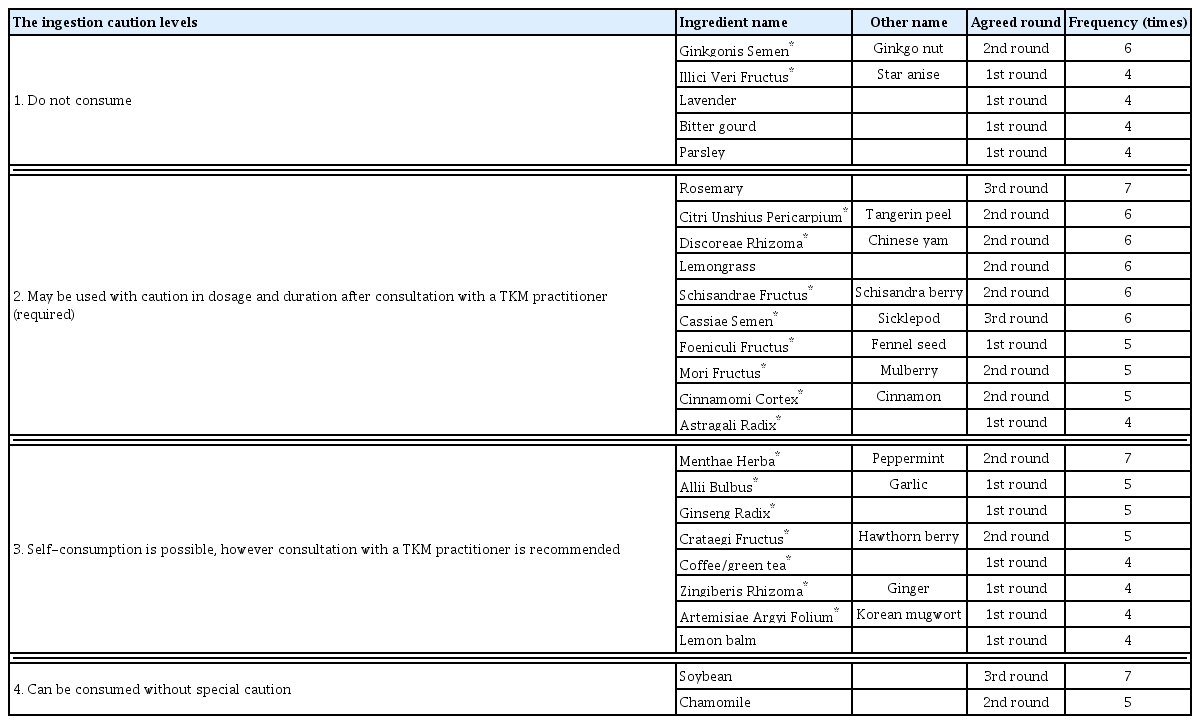
In the first round of Delphi, 12 of the 25 ingredients resulted in consensus, and the second round of Delphi was conducted for the 13 disagreed ingredients that did not meet the consensus threshold, resulting in additional consensus for 10 ingredients. The remaining three ingredients—soybean, rosemary, and Cassiae Semen—reached consensus in the third round of Delphi (Figure 1,2). Consequently, a consensus was reached for 5 ingredients at level 1 advisory (Do not consume), 10 ingredients at level 2 advisory (May be used with caution in dosage and duration after consultation with a TKM practitioner (required)), 8 ingredients at level 3 advisory (Self-consumption is possible, however consultation with a TKM practitioner is recommended), and 2 ingredients at level 4 advisory (Can be consumed without special caution) (Table 3).
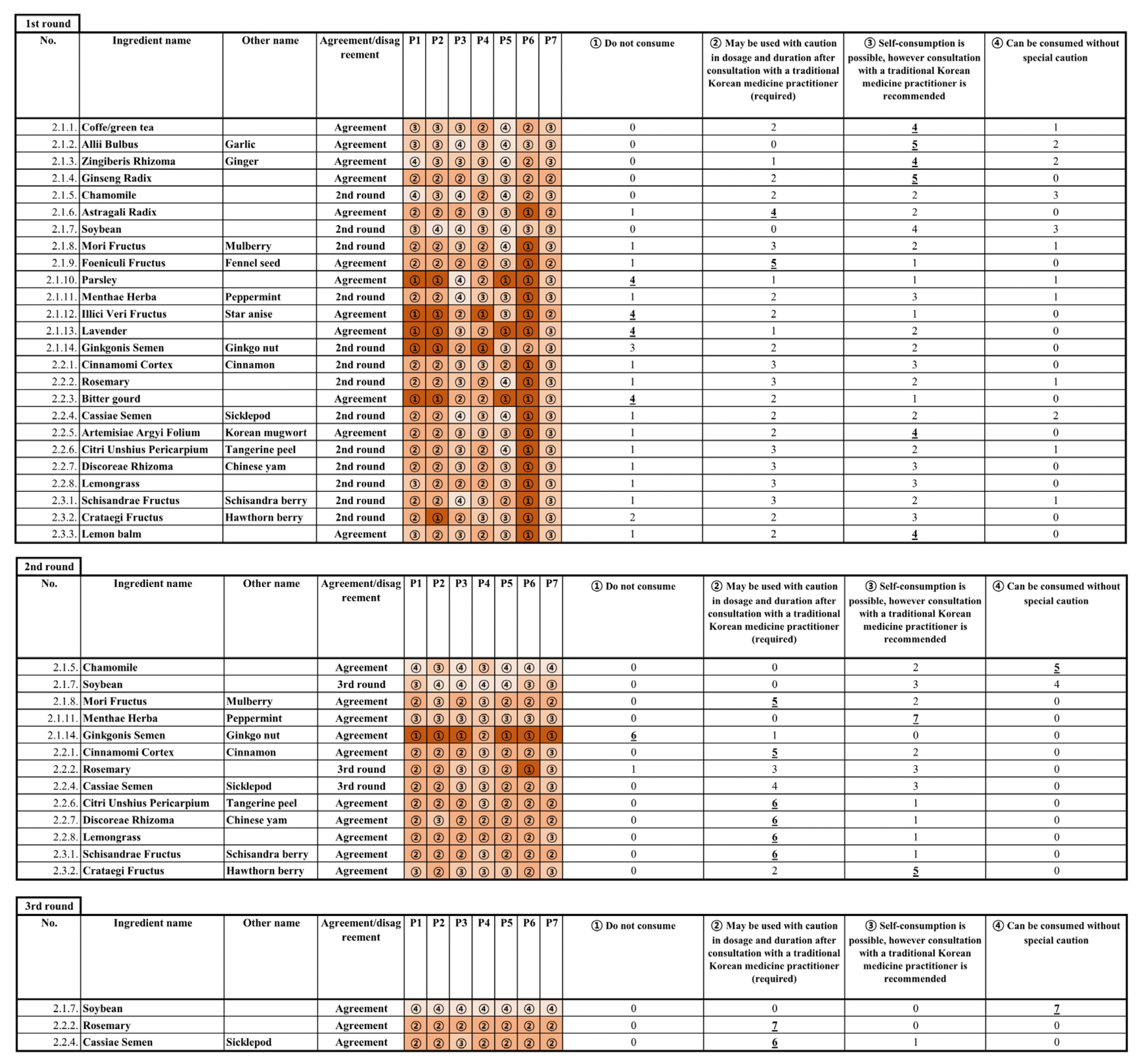
Records of each Delphi expert consensus process and distribution of panelist responses. The circled numbers in each panel represent the ratings, and a bold, underlined number means it was selected and agreed upon with that frequency of response.
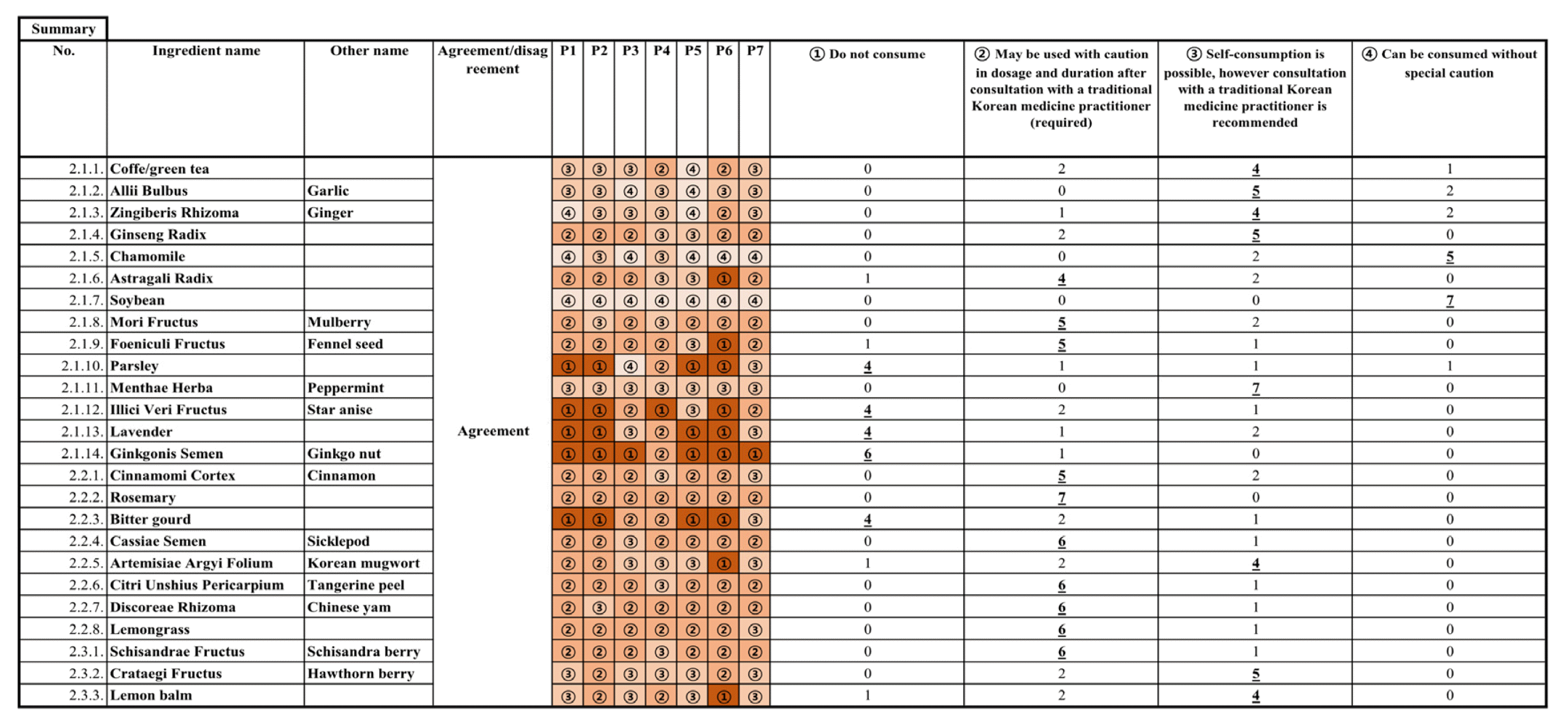
Summary of final consensus results and distribution of panelists responses. The circled numbers in each panel represent the ratings, and a bold, underlined number means it was selected and agreed upon with that frequency of response.
Analysis of opinions gathered during the three rounds of expert consensus revealed that panelists seemed to have perceived greater risk for ingredients included in the questionnaire when the words “toxicity,” “hormone,” “deformity,” “miscarriage,” and “epileptic seizure” were used, irrespective of evidence level presented in the questionnaire.
Discussion
In East Asia, where traditional medicine using medicinal herbs is practiced, Korea has a very high percentage of medicinal herbs for food use compared to China, Japan, and Taiwan, and there has always been a risk of mistaking medicinal herbs, which are highly medicinal in nature1). In addition, various countermeasures have been raised in response to concerns about possible public health hazards caused by indiscriminate use as an ingredient in health functional foods1,6).
In Korea, safety studies for fetus during pregnancy have been conducted on prescribed herbal medicine by TKM practitioners, however safety studies have not been conducted on medicinal herbs for food use and pure food ingredients14,15). Therefore, we conducted expert Delphi consensus to assess the potential risks to the fetus during pregnancy of these medicinal herbs for food and pure food ingredients and to develop a minimum level of information that can be provided to the general public. We collected and summarized the literature on fetal safety for 25 ingredients in medicated diet recipes, developed a Delphi consensus questionnaire with the ingestion caution levels, and asked expert panelists to respond.
Ginkgonis Semen (Ginkgo nut), Illici Veri Fructus (Star anise), lavender, bitter gourd, and parsley were agreed at the level 1 of “Do not consume”. The dangers of O-methylpyridoxine of Ginkgo nut, a neurotoxin, have been well documented, with reports of adverse effects from overdose, even when baked, and a case of a 2-year-old child experiencing a tonic-clonic seizure after ingestion16,17). Expert opinion gathered during the Delphi also recognized the well-known risk of Ginkgo nut toxicity. Illici Veri Fructus has been used in a variety of foods and teas, and traditionally, anise oil has been used as an antispasmodic and labor inducer, with case series of tonic-clonic epileptic seizures reported in infants18,19). Delphi panelists were not inclined to recommend the use of star anise, which is not an essential component of recipe or herbal medicine. Despite the low quality of evidence from known studies including case series reporting controversial result because of the lack of enough information, lavender appeared to have a high perceived risk owing to its hormonal activity and traditional usage as an emmenagogue and abortifacient18,20). Animal studies with pregnant rats or mice have shown the teratogenic risk of bitter gourd seed, however the decision was made based on the possibility that the seeds could be consumed in combination with the pulp21). Parsley, another herb frequently used as a traditional abortifacient, was also deemed high risk owing to reports of seven miscarriages by oral ingestion and injection into the body, the hormonal actions of the extract, and the mutagenic potential of the myristicin component22,23,24).
The following 10 ingredients were recognized as being closer to medicinal herb than food ingredient and were agreed at the level 2 of “consultation with TKM practitioner is required”: Rosemary, Citri Unshius Pericarpium, Discoreae Rhizoma, lemongrass, Schisandrae Fructus, Cassiae Semen, Foeniculi Fructus, Mori Fructus, Cinnamomi Cortex, and Astragali Radix. The questionnaire included the results of a retrospective clinical study of resveratrol, the main component in Mori Fructus and the risks reported in animal studies of the extracts and main components of the other five herb and food ingredients. Schisandrae Fructus and Cinnamomi Cortex had only reported risks from literature review studies. Astragali Radix has been reported to be safe in randomized clinical trials with intravenous injection of extract, however these results should not be interpreted as equivalent to the risks of oral ingestion25,26). When Astragali Radix, Citri Unshius Pericarpium and Discoreae Rhizoma were administered at 20g/day, 9g/day, and 15g/day, respectively, during the organogenesis period of pregnant mice, Astragali Radix showed a significant increase in postnatal death, Citri Unshius Pericarpium in fetal resorption, postnatal death, and late death, and Discoreae Rhizoma in polydactyly, postnatal death, and early death compared to the control27). In an observational study of 630 mothers within 3 days postpartum, gestational age was significantly but marginally shorter in the group which used Foeniculi Fructus during pregnancy compared to non-use group28). A retrospective data analysis of 3,060 pregnant women who conceived via in vitro fertilization found that women who consistently consumed resveratrol, the main ingredient in Mori Fructus, had a higher risk of miscarriage (OR 2.602; 95%CI 1.070–6.325)29). Cinnamomi Cortex extract showed a concentration-dependent reduction in contraction on isolated rat uterine strips, demonstrating action on the myometrium30). Citral and myrcene, the main components of lemongrass, have been reported to cause birth defects in rats31,32). A clinical trial covered in the literature review reported that a 3-day administration of Schisandrae Fructus 70% ethanol-derived tinc caused induction of labor and increased labor induction in multipara18). Rosemary water extract has been reported to cause an increased number of anomalous embryos and prolonged embryo retention in pregnant rats33). Reduced fetal weight was reported when emodin, a major component of Cassiae Semen, was administered to pregnant mice34).
Factors that may have influenced the expert panelist’s decision-making process in reaching an overall consensus included the degree of overdose potential in the domestic environment, the frequency of use as an ingredient in herbal medicines or as a food, and the risk in the first trimester of pregnancy. We also estimated that ingredients with more perceived risk terms in the literature summaries, such as “toxicity,” “hormones,” “malformations,” “miscarriage,” and “epileptic seizures,” were more likely to had a higher risk rating by panelist.
In some cases, each panel’s previous questionnaire response changed in the next round. We interpreted this phenomenon as a shift in thinking because the results of the Delphi responses and opinions were shared with the panelists, therefore they could see and compare their own responses and opinions within the overall responses again. This sharing process has the effect of forcing panelists to rethink their decisions from a different angle, leading to clearer judgements35).
We suspected that the expert panelists were most concerned with the gap between expert and public views in their assessment and rating, as the public use of medicinal herbs for food is a sensitive topic. An example is the opposition that might be expected if the public were advised to consume a commonly used ingredient after a mandatory consultation with a TKM practitioner. Another example is the concern from the TKM practitioner community when a recommendation is made that certain medicinal herbs can be self-administered by the public. On the other hand, the expert group’s assessment of “consultation with TKM practitioner is required” for ingredients such as Astragali Radix, Cinnamomi Cortex, Citri Unshius Pericarpium, Discoreae Rhizoma, Rosemary, lemongrass, Schisandrae Fructus, and Cassiae Semen, which have been frequently used in food in Korea, is expected to be new information for the public.
The significance of this study is that it derived new results through expert consensus on medicinal herbs for food and pure food ingredients, which has not been conducted in Korea to date. Moreover, the consensus on relatively conservative safety assessments for various ingredients used for food is a significant step toward expanding the scope of TKM experts’ involvement in public health and ensuring fetal safety in the daily life. In addition, we believe that this study addressed “whether and to what extent TKM practitioners are involved in intake of medicinal herbs for food,” and that it also has developmental implications that could lead to specific discussions in the future.
This study has several limitations, including the following. First, because of the ambiguity inherent in the medicinal herbs for food, each panel’s internal judgement of risk seemed inconsistent across the entire Delphi process. Factors that contributed to ambiguity included the ease of consumption by the general public, the history of unchecked consumption as an everyday food ingredient, and the anticipated public reactions to agreed caution levels. Second, the significant paucity of published clinical studies on adverse effects of medicinal herbs for food and pure food ingredients may have affected the quality of the Delphi questionnaire and consensus results. Third, this study did not perform an intensive risk discussion focused on the first trimester, which poses the highest fetal risk. Fourth, because we used a relatively small number of expert panelists compared to a typical Delphi study, the result of this study may not have included a sufficiently diverse range of perspectives.
This study conducted an expert Delphi consensus on ingredients that are readily consumed by pregnant women and have a relatively high number of reported risks for the fetus during pregnancy. Further studies should be conducted according to the trimester of pregnancy, and intensive research should be conducted on medicinal herbs for food, which are recognized as specialized herbal medicines rather than pure food ingredients by TKM practitioners. Based on the results of the Delphi consensus, we expect to be able to provide the general public with relatively evidence-based fetal safety information on medicinal herbs for foods that are on the borderline between herbal medicine and food in Korea, as well as food ingredients that are easy for pregnant women to consume.
Conclusion
Based on the consensus of a seven-member expert panel of TKM gynecologists, Ginkgonis Semen (Ginkgo nut), Illici Veri Fructus (Star anise), lavender, bitter gourd, and parsley should be avoided by pregnant women. For rosemary, Citri Unshius Pericarpium, Discoreae Rhizoma, lemongrass, Schisandrae Fructus, Cassiae Semen, Foeniculi Fructus, Mori Fructus, Cinnamomi Cortex, and Astragali Radix, the level 2 advisory may be recommended to use with caution and to consult a TKM practitioner for advice on consumption, dose, and duration. Menthae Herba (peppermint), Allii Bulbus, Ginseng Radix, Crataegi Fructus, Coffee or green tea, Zingiberis Rhizoma, Artemisiae Argyi Folium, Lemon balm are agreed to be relatively safe to consume and may be recommended with the level 3 advisory, with a recommendation to consult a TKM practitioner if necessary.
Acknowledgement
This work was supported by the Collection of Clinical Big Data and Construction of Service Platform for Developing Korean Medicine Doctor with Artificial Intelligence research project (grant number KSN2021110).