References
1. Korean Academy of Medical Sciences. Evidence-based Chronic Kidney Disease Clinical Practice Guidelines for Primary Medical Use Seoul: Korean Academy of Medical Sciences & Korea Disease Control and Prevention Agency; 2022. [cited 2023 June 19]. Available from: ULR:
https://www.ksn.or.kr/bbs/?code=g_guideline
.
3. Portolés J., Martín L., Broseta J.J., Cases A.. 2021;Anemia in chronic kidney disease: from pathophysiology and current treatments, to future agents. Frontiers in Medicine 8:642296.
https://doi:10.3389/fmed.2021.642296
.
4. Santos E.J.F., Filho N.S., Dos Santos A.. 2020;Erythropoietin resistance in patients with chronic kidney disease: current perspectives. International journal of nephrology and renovascular disease 13:231–237.
https://doi:10.2147/IJNRD.S239151
.
5. Mimura I., Tanaka T., Nangaku M.. 2015;How the target hemoglobin of renal anemia should be? Nephron 131(3):202–209.
https://doi:10.1159/000440849
.
6. Kliger A.S., Foley R.N., Goldfarb D.S., Goldstein S.L., Johansen K., Singh A., et al. 2013;KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. American Journal of Kidney Diseases 62(5):849–859.
https://doi:10.1053/j.ajkd.2013.06.008
.
7. Singh A.K.. 2010;What is causing the mortality in treating the anemia of chronic kidney disease: erythropoietin dose or hemoglobin level? Current Opinion in Nephrology and Hypertension 19(5):420–424.
https://doi:10.1097/MNH.0b013e32833cf1d6
.
10. Zheng L., Liu M., Sun X.. 2023;Analysis of medication rules of traditional Chinese medicine in treatment of renal anemia based on data mining (基于数据挖掘的中药治疗 肾性贫血的用药规律研究). China Pharmacy 34(05):591–594+619.
https://doi:10.6039/j.issn.1001-0408.2023.05.15
.
11. Zhao M., Zhang Y., Li L., Yu Z., Li B.. 2017;Efficacy and safety of Danggui Buxue Decoction in combination with western medicine treatment of anemia for renal anemia: a systematic review and meta-analysis. Annals of Translational Medicine 5(6):136.
https://doi:10.21037/atm.2017.01.17
.
12. Park J.K., Kim K.H.. 2017;A Survey on Uncovered Services in National Health Insurance of Traditional Korean Medicine Institution. Journal of Society of Preventive Korean Medicine 21(3):43–50.
https://doi:10.25153/spkom.2017.21.3.005
.
13. Chen W.D., Huang H.S., Su Y.C., Chou S.C., Ho W.C., Kao M.C., et al. 2018;The characteristics and prescription patterns of Chinese herbal medicine in clinical practice for the treatment of anemia. Taiwanese Journal of Obstetrics and Gynecology 57(4):570–577.
14. Faculty of Internal Medicine of Liver System of Korean Medicine. 2016. Textbook of Internal Medicine of Liver System in Korean Medicine Seoul: Book Publisher Nadoh. p. 540.
15. East-West Medical Convergence Research Society. 1997. East-West Clinical Medicine Seoul: Younglim Publishing Company. p. 113–116.
16. Chen L.J., Yu Y., Xiong W.S.. 2019;A Meta-analysis of Randomized Controlled Trials of Guipi Decoction in Assisting Treatment of Iron Deficiency. Anemia Journal of Zhejiang University of TCM 43(12):1368–1376.
https://doi:10.16466/j.issn1005-5509.2019.12.015
.
17. Yang M., Geng S., Zhu Y., Fei Z., Li X., Zhang J., et al. 2020;Meta-analysis of Guipi Decoction Combined with Western Medicine on Iron Deficiency Anemia. Clinical Journal of Chinese Medicine 15:49–52+55.
https://doi:doi:10.3969/j.issn.1674-7860.2020.15.021
.
18. Li J., Cai Y.. 2010;Thirty cases of megaloblastic anemia treated by Guipi decoction combined with Western medicine (归 脾汤联合西药治疗巨幼细胞贫血30例). Shaanxi Journal of Traditional Chinese Medicine 31(10):1347–1348.
19. Dong L., Liu H., Gu Q., Han Z.. 2015;Effect of Guipi Decoction on Bone Marrow Serum FGF-1 in Patients with Chronic Aplastic Anemia of Heart and Spleen Deficiency. China Tranaslational Medicine and Integrative Medicine Symposium (Guangzhou station) Papers Comprehensive Journal Chinese Journal of Hypertension Publishing Society Proceedings :2.
20. Yang Y.. 2020;Clinical observation of Guipi decoction combined with ferrous sulfate folic acid tablets in the treatment of perioperative anemia in elderly patients with intertrochanteric fractures (归脾汤结合硫酸亚铁叶酸片治疗老年股 骨转子间骨折围手术期贫血的临床观察). Nei Mongol Journal of Traditional Chinese Medicine 39(05):69–71.
https://doi:10.16040/j.cnki.cn15-1101.2020.05.043
.
21. Wang C., Li T.. 2020;Efficacy evaluation of traditional Chinese medicine for nourishing qi, nourishing blood and promoting blood circulation in the treatment of myelodysplastic syndrome-refractory anemia (益气养血活血中药 治疗骨髓增生异常综合征-难治性贫血的疗效评价). Guide of China Medicine 18(07):182–183.
https://doi:10.15912/j.cnki.gocm.2020.07.136
.
22. Liu X., Su L., Zhu X.. 2019;Clinical Study on Modified Guipi Tang Combined with Routine Therapyfor Cirrhosis Complicated with Anemia. Journal of New Chinese Medicine 51(05):76–78.
https://doi:10.13457/j.cnki.jncm.2019.05.022
.
24. Li S.. 2020;Clinical Study of Jiawei Guipi Decoction in Treating Tumor-Related Anemia in Patients with Gastric Cancer. Anhui University of Traditional Chinese Medicine. Master's thesis
https://doi:10.26922/d.cnki.ganzc.2020.000023
.
25. Bao Y., Zhou H., Ni Y., Qian H.. 2023;Clinical Study on Guipi Decoction Combined with Roxadustat for Chronic Kidney DIsease Complicated with Renal Anemia. New Chinese Medicine 55(09):64–67.
https://doi:10.13457/j.cnki.jncm.2023.09.011
.
26. Federation of Herbal Formula Professors of Colleges of Korean Medicine. 2019. Textbook of Herbal Formula Seoul: Yeonglimsa. p. 290–291.
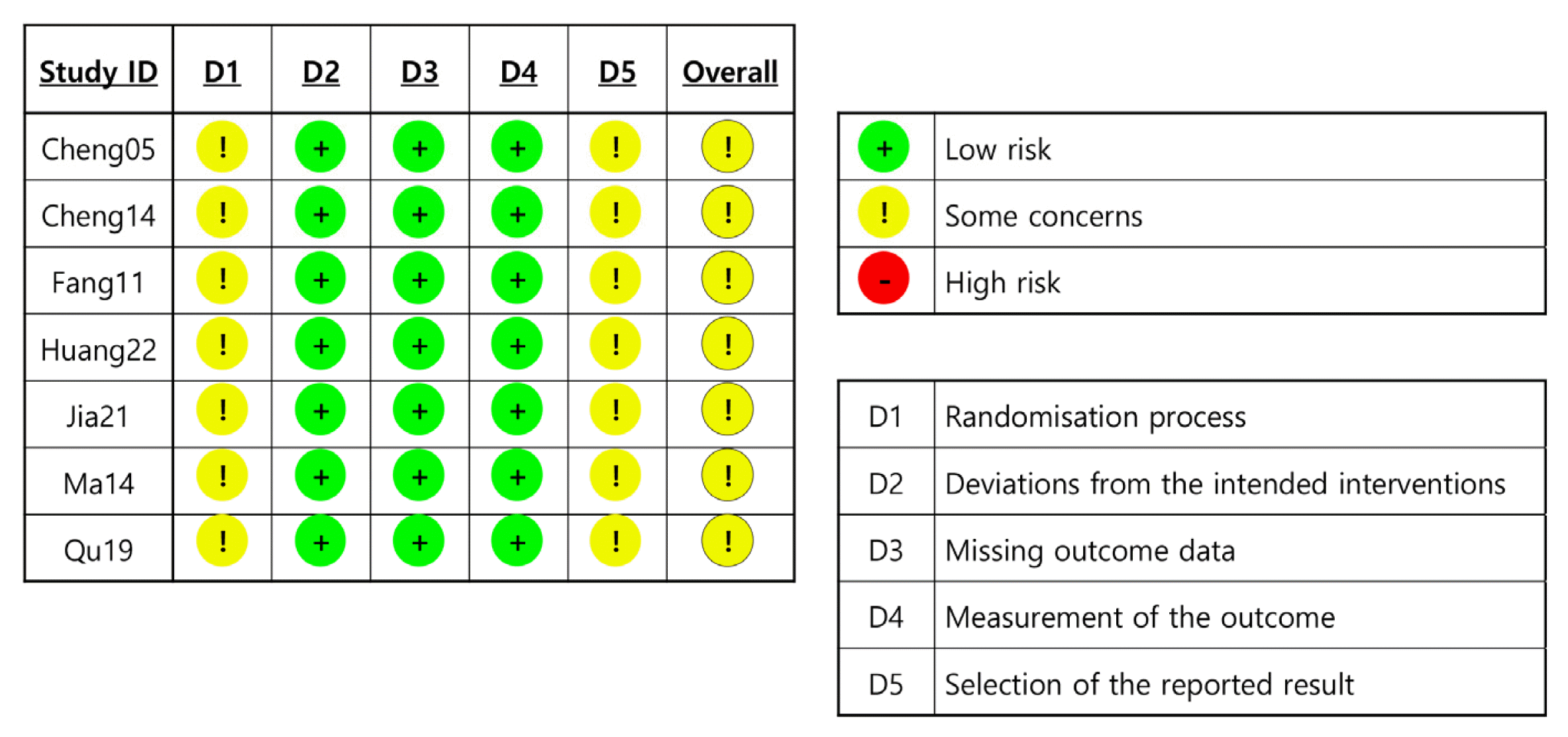
29. Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. 2019;RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj 366:l4898.
https://doi:10.1136/bmj.l4898
.
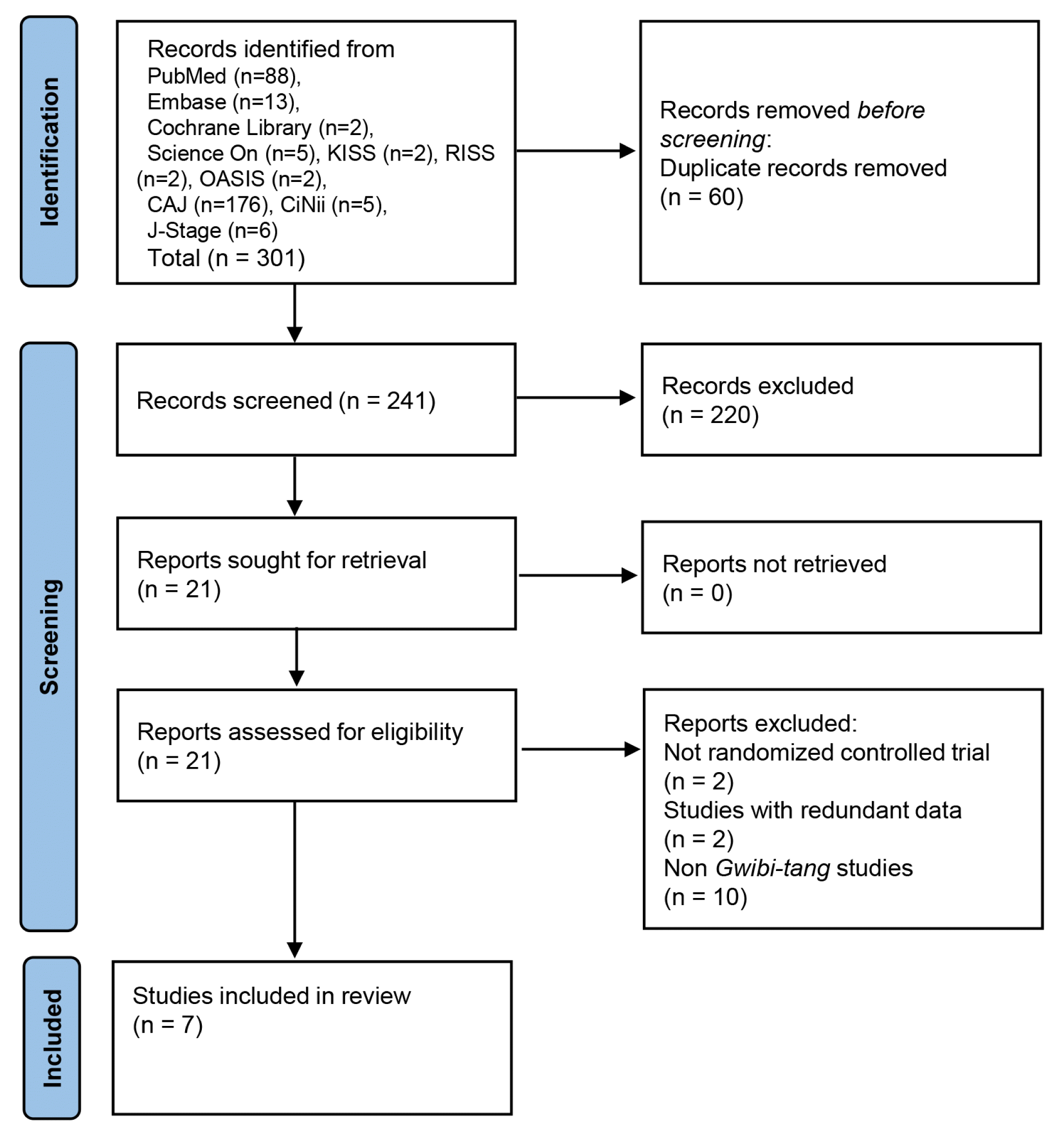
30. National Evidence-based Healthcare Collaborating Agency. 2022. Handbook for Clinical Practice Guideline Developer Seoul: NECA. p. 135–143.
p. 343–347.
31. Higgins J.P.T., Savović J., Page M.J., Elbers R.G.. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 2022. [cited 2023 June 19]. Available from: URL:
www.training.cochrane.org/handbook
.
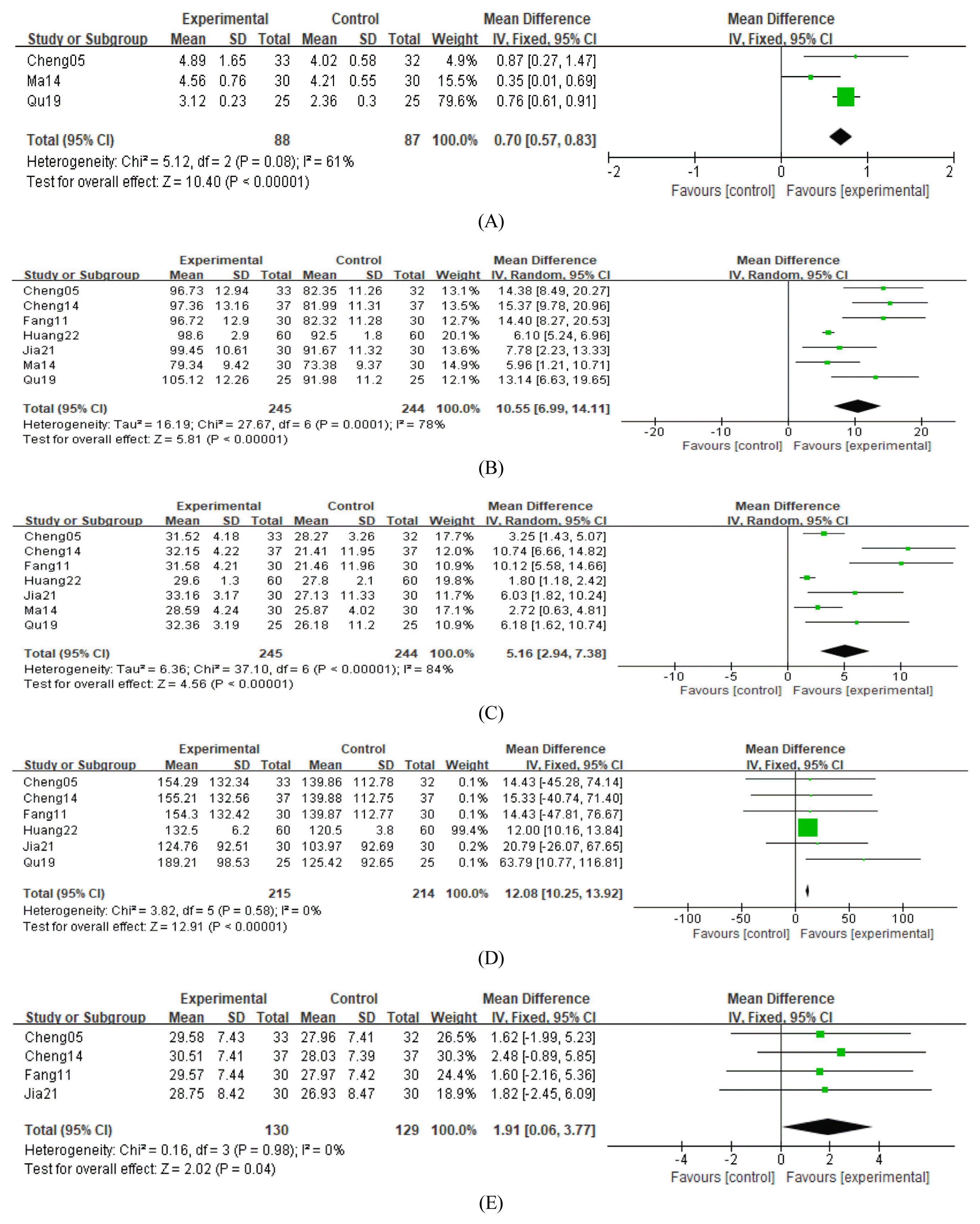
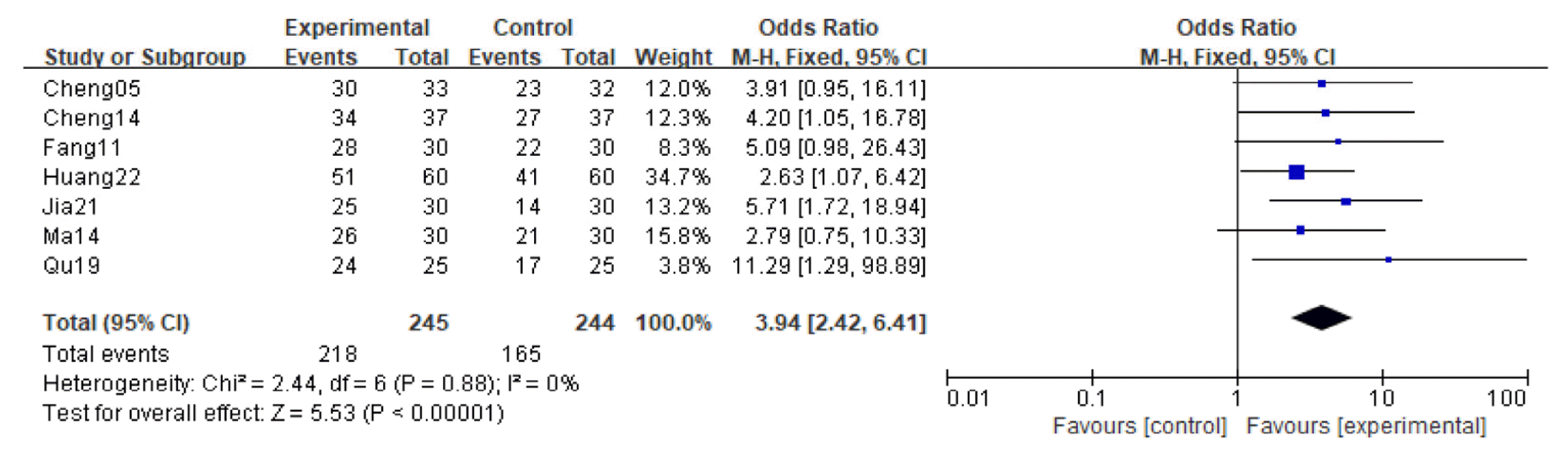
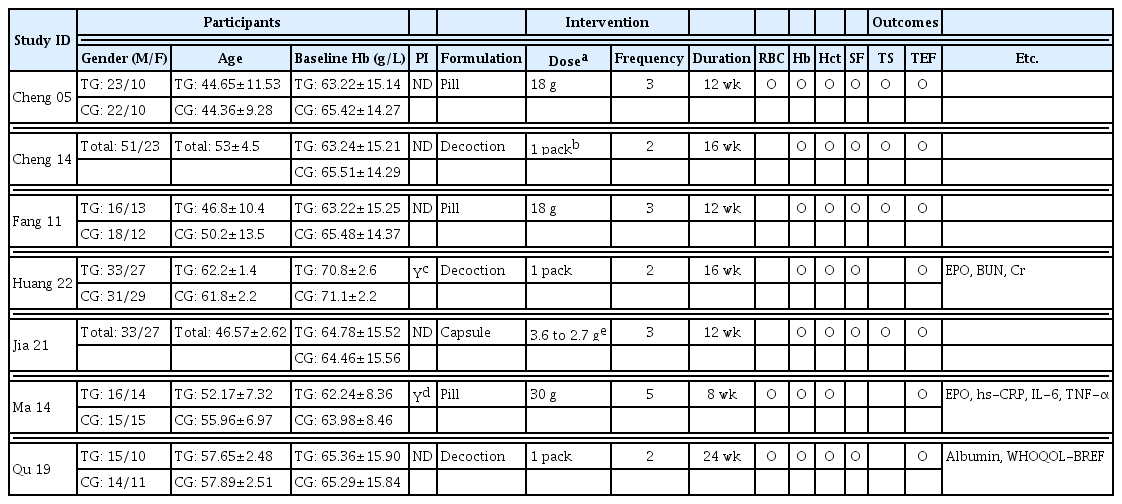
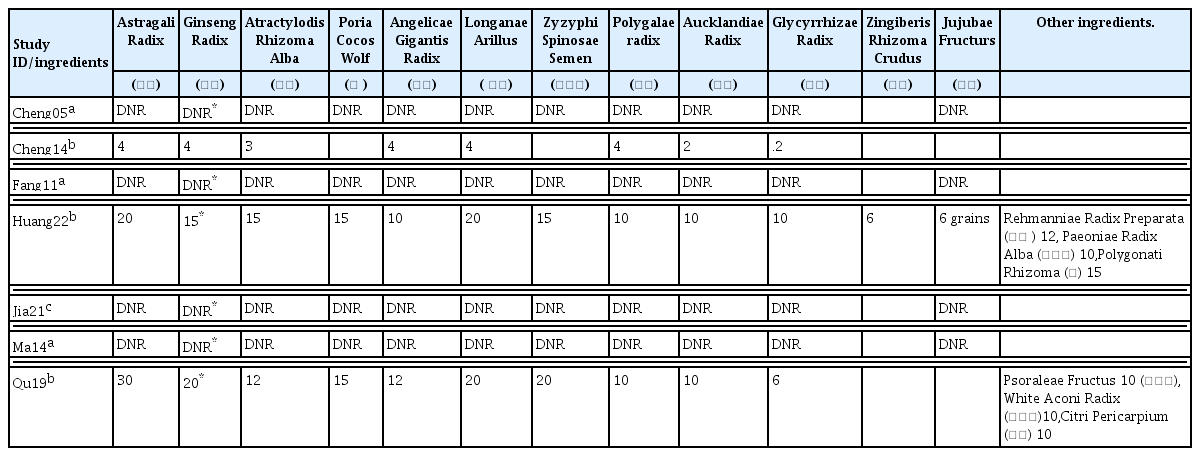
32. Cheng J., Yi W., Pan X., Qi A., Cheng S.. 2005;Efficacy of GuiPiWan Treating Anemia Patients During Hemodialysis. Journal of Nursing Science 20(01):41–42.
33. Cheng X., Huang X., Yao L.. 2014;Clinical Efficacy Analysis of Guipi Decoction in the Treatment of Anemia in Patients undergoing. Hemodialysis 9(11):1482–1484.
34. Fang H., Wu G., Xu W., Fu S.. 2011;Curative Effect Observation on 30 Cases of Maintenance Hemodialysis Anemia Treated by Guipi Pill (归脾丸治疗维持性血液透析贫血30例 疗效观察). Zhejiang Journal of Traditional Chinese Medicine 46(06):423–424.
35. Huang T., Li X., Guo T.. 2022;Effect of modified Guipi decoction on renal anemia and its effect on erythropoietin level (归脾汤加减治 疗肾性贫血的效果及对促红细胞生成素水平的影 响). Heilongjiang Medicine Journal 35(04):847–849.
https://doi:10.14035/j.cnki.hljyy.2022.04.037
.
36. Jia Z., Wang L., Zhou L., Cai X.. 2021;Improvement effect of Guipi Capsule on erythropoietin resistance in maintenance hemodialysis patients (归脾胶囊对维持性血液透 析患者促红细胞生成素抵抗的改善作用). Chinese Journal of Traditional Medical Science and Technology 28(06):946–947.
37. Ma J., Zhang S.. 2014;Discussion on “Enriching Blood by Tonifying Spleen” Connotation from Adjusting of Micro-inflammatory State in Renal Anemia Patients with MHD Technique Interfering by Gui-Pi Pills. Modernization of Traditional Chinese Medicine and Materia Medica-WOrld Science and Technology 16(03):634–647.
38. Qu W.. 2019;Observation on the application effect of traditional Chinese medicine Guipi decoction in patients with uremia hemodialysis anemia (中药归脾汤在尿毒症血液透析贫血患者中 的应用效果观察). Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine 2019;19(03):82–4.
https://doi:10.13638/j.issn.1671-4040.2019.03.041
.
39. Reinisch W., Staun M., Tandon R.K., Altorjay I., Thillainayagam A.V., Gratzer C., et al. 2013;A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). American Journal of Gastroenterology 108(12):1877–1888.
https://doi:10.1038/ajg.2013.335
.
40. Ministry of Food and Drug Safety. 2011. Guidelines for Non-Clinical and Clinical Evaluation of Biosimilars of Erythropoietin Osong: MFDS.
41. Deved V., Poyah P., James M.T., Tonelli M., Manns B.J., Walsh M., et al. 2009;Ascorbic acid for anemia management in hemodialysis patients: a systematic review and meta-analysis. American journal of kidney diseases 54(6):1089–1097.
https://doi:10.1053/j.ajkd.2009.06.040
.
42. Ryu S.R., Park S.K., Jung J.Y., Kim Y.H., Oh Y.K., Yoo T.H., et al. 2017;The prevalence and management of anemia in chronic kidney disease patients: result from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD). Journal of Korean medical science 32(2):249–56.
https://doi:10.3346/jkms.2017.32.2.249
.
43. Evans M., Bower H., Cockburn E., Jacobson S.H., Barany P., Carrero J.J.. 2020;Contemporary management of anaemia, erythropoietin resistance and cardiovascular risk in patients with advanced chronic kidney disease: a nationwide analysis. Clinical kidney journal 13(5):821–827.
https://doi:10.1093/ckj/sfaa054
.
44. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. 2011 Geneva: World Health Organization (WHO/NMH/NHD/MNM/11.1); 2016. [cited 2023 June 19]. Available from:
http://www.who.int/vmnis/indicators/haemoglobin.pdf
.
45. Pfeffer M.A., Burdmann E.A., Chen C.Y., Cooper M.E., De Zeeuw D., Eckardt K.U., et al. 2009;A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. New England Journal of Medicine 361(21):2019–2032.
https://doi:10.1056/NEJMoa0907845
.
47. Pollock C., Johnson D.W., Ho W.H., Rossert J., Casadevall N., Schellekens H., et al. 2008;Pure red cell aplasia induced by erythropoiesis-stimulating agents. Clinical Journal of the American Society of Nephrology 3(1):193–199.
https://doi:10.2215/CJN.02440607
.
48. HIRA. Details on application standards and methods of medical care benefits (drugs). Lucy design July. 2022;edition.
50. Li L.. 2020;Clinical Observation on Modified Guipi Decoction Combined with Erythropoietin in Treating Anemia in Patients with Maintenance Hemodialysis (加减归脾汤联 合促红细胞生成素治疗维持性血液透析患者贫血的 临床观察). Health for Everyone 12:104.
51. Zhou X.. 2017. Clinical Study of the Effect of Guipi Decoction on Bone Marrow Suppression after Chemotherapy for NSCLC, Hubei University of Traditional Chinese Medicine. Master's thesis
52. 殷丽娟 刘立, 许瑞 彭晓明, 杨欢 . 归脾汤对苯中 毒小鼠骨髓造血干细胞表型 Sca-1 和 CD34+, 细 胞分裂周期的影响. 北京中医药大学学报 2014;37(4):255–8.
53. 李龙龙 刘立, 高丽娟 周亚兰. 归脾汤对苯中毒小 鼠外周血, 骨髓有核细胞及细胞凋亡蛋白 Fas, FasL 表达的影响. 中医临床研究 2018;10(3):31–5.
54. Chang M.S., Kim D.R., Ko E.B., Choi B.J., Park S.Y., Kang S.A., et al. 2009;Treatment with Astragali radix and Angelicae radix enhances erythropoietin gene expression in the cyclophosphamide-induced anemic rat. Journal of Medicinal Food 12(3):637–642.
https://doi:10.1089/jmf.2007.0727
.
55. Zhang L., Gong A.G., Riaz K., Deng J.Y., Ho C.M., Lin H.Q., et al. 2017;A novel combination of four flavonoids derived from Astragali Radix relieves the symptoms of cyclophosphamide-induced anemic rats. FEBS Open Bio 7(3):318–323.
https://doi:10.1002/2211-5463.12146
.
56. Jang E., Kwon S., Cho Y., Woo H., Kim Y., Lee J.. 2013;A case of Guibitanggami treatmentto an iron deficiency anemia patient. The Journal of Internal Korean Medicine spr. (1):142–147.
57. Pyeon B.. –2012.
Anemia correction in patients with chronic renal failure (administration of hematopoietic hormones). Journal of Korean Society of Health-System Pharmacists 29(1):87–94.
58. Li J., Ha H., Jung D., Lee H., Lee J., Huang D., Shin H., et al. 2010;Comparative Study of 25 Herbal Formulas on Anti-Inflammatory Effect. The Jorunal of Oriental Obstetrics & Gynecology 23(3):101–111.
59. Shin N.R., Jung T.Y., Seo C.S., Park S.W., Ko J.W., Kim J.C., et al. 2018;Protective effect of water extract of guibi-tang against pulmonary inflammation induced by cigarette smoke and lipopolysaccharide. Laboratory Animal Research 34:92–100.
https://doi:10.5625/lar.2018.34.3.92
.
60. Kim J., Lee J., Ha H., Seo C., Lee M., Lee H., et al. 2009;Analysis of Studies on Guibi-tang (Guipitang) for Fundamental Establishment of Evidence Based Medicine. Journal of oriental neuropsychiatry 20(3):205–216.
61. Gluba-Brzózka A., Franczyk B., Olszewski R., Rysz J.. 2020;The Influence of Inflammation on Anemia in CKD Patients. International Journal of Molecular Sciences 21(3):725.
https://doi:10.3390/ijms21030725
.
62. Dai C., Shi L., Ji Y., Xia L., Jiang Y., Zhang A., et al. 2019;Clinical study of the effects of guipi decoction on oxygen carry capacity of red blood cells in the patients with anemia of heart and spleen deficiency. World Journal of Integrated Traditional and Western Medicine 9:1283–1287.
https://doi:10.13935/j.cnki.sjzx.190924
.
63. Rong Y., Wu D., Li M., Teng J.. 2023;Efficacy and safety of Guipi Decoction in the treatment of chronic heart failure: A systematic review and meta-analysis of randomized controlled trials. Medicine 102(9):e33181.
https://doi:10.1097/MD.0000000000033181
.
64. Hwang J., Yang M., Shin H.. 1997;Survey for approximate composition and mineral content of medicinal herbs. Korean Journal of Food Science and Technology 29(4):671–9.
65. Lee J.. 2007. A Study on effectiveness of dietary self-efficacy, dietary knowledge and social support for exercise diet compliance of patients on hemodialysis. Yonsei University; Master's thesis
66. Chang D., Zhang G.. 2019;Curative effect of modified Guipi decoction on renal anemia in patients with chronic kidney disease and its influence on blood routine and blood biochemical indicators (加味归脾汤治疗慢性肾 脏病患者肾性贫血的疗效及对血常规和血生化指标 的影响). Journal of North Pharmacy 16(04):104–105.
67. Qiu S.. 2021;Application effect of modified Guipi decoction combined with erythropoietin in the treatment of anemia in patients with maintenance hemodialysis (加减归脾汤联合促红 细胞生成素在维持性血液透析患者贫血治疗中的应 用效果). Clinical Research 29(08):128–9.
68. Lee J., Eum H., Chung T., Lee Y., Um Y., Yim N., et al. Single Dose Oral Toxicity Study of the Gwibi-tang Extract in ICR Mice. The Journal of Oriental Obstetrics Gynecology 23(4):47–56.
69. Lee M., Seo C., Kim Y., Shin H.. 2015;Safety assessment of Guibi-tang: Subchronic toxicity study in Crl: CD SD rats. Regulatory Toxicology and Pharmacology 73(2):485–493.
70. Ministry of Food and Drug Safety. 2011. Information Book for Proper Use of Drugs for Patients with Renal Diseases Seoul: Ministry of FDS. p. 20.