References
2. Ministry of Food and Drug Safety. 2023;Regulations on the safety of Pharmaceuticals etc (No. 1848)
3. Ministry of Food and Drug Safety. 2022;Toxicity test standards for pharmaceuticals etc (No.2022–18)
4. Ministry of Food and Drug Safety. 2021;Regulations on notification of product approval for traditional herbal medicine & herbal medicine preparations etc (No. 2021–38)
5. Lee S.D., Kwang S.S.. 1991;A Toxicity Study of Herbal Drugs. The journal of Won kwang Oriental Medicine 1(1):33–38.
6. Ministry of Food and Drug Safety. 2022;Non-clinical test management standard etc (No. 2022–93)
7. Shin H.K., Ha H.K., Seo C.S., Lee M.Y.. 2018. Standard herbal medicine formulary Korea institute of oriental medicine. p. 1–3.
9788959703791.
8. National Institute of Food and Drug Safety Evaluation. 2021;Toxicity test method guideline for foods, etc. Single dose toxicity test method :1–51.
9. Lee S.D.. 1999;Toxic Concept in Oriental Medicine. J Society Prevent Korean Med 3(1):157–172.
10. Chang I.K., Hong N.D.. 1985;Experimental studies on the acute toxicity and the efficacy of Phyllostachys juice. J Int Korean Med 2(1):83–101.
11. Cho S.H., Lee Y.H., Park Y.B.. 1992;Acute Toxicity Study of Aqua-acupuncture of Cervi Cornu Parvum and Ganoderma Lucidum Karsten in Mice. The Journal of Korean Acupuncture & Moxibustion Society 9(1):71–83.
12. Choi M.K., Lee Y.H.. 1992;Studies on the Safety assessment of Cervus elaphus Extract for Aqua-acupuncture. The journal of Kyung Hee Oriental Medicine 15(1):203–229.
13. Lee H.J.. 1993;Study for the Toxicity of Kinds of Ginseng Radix Aqua-acupuncture Extract. The Journal of Korean Acupuncture & Moxibustion Society 10(1):167–173.
14. Nam Y.S., Lee Y.H.. 1996;Studies on the Safety Assessment of Red-ginseng Radix Extract Solution for Herb-acupuncture. J korean Medicine 17(1):478–493.
15. Park M.R.. 1995. Studies on the Acute oral toxicity of Sojeokbaekchoolsan in mice Taejeon university. p. 1–39.
16. Kim Y.C., Lee J.H., Lee H.J.. 1997;Studies on oral toxicity of Inchinchunggan-tang in mice. The journal of Kyung Hee Oriental Medicine 20(1):57–89.
17. Yong K.H.. 1985;Prospect of good laboratory practice in Korea. Korean J. of Toxicology 1(1):43–48.
18. Park G.L.. 2005;Meaning and perspective of GLP. The Monthly Technology & Standards 39:93–96.
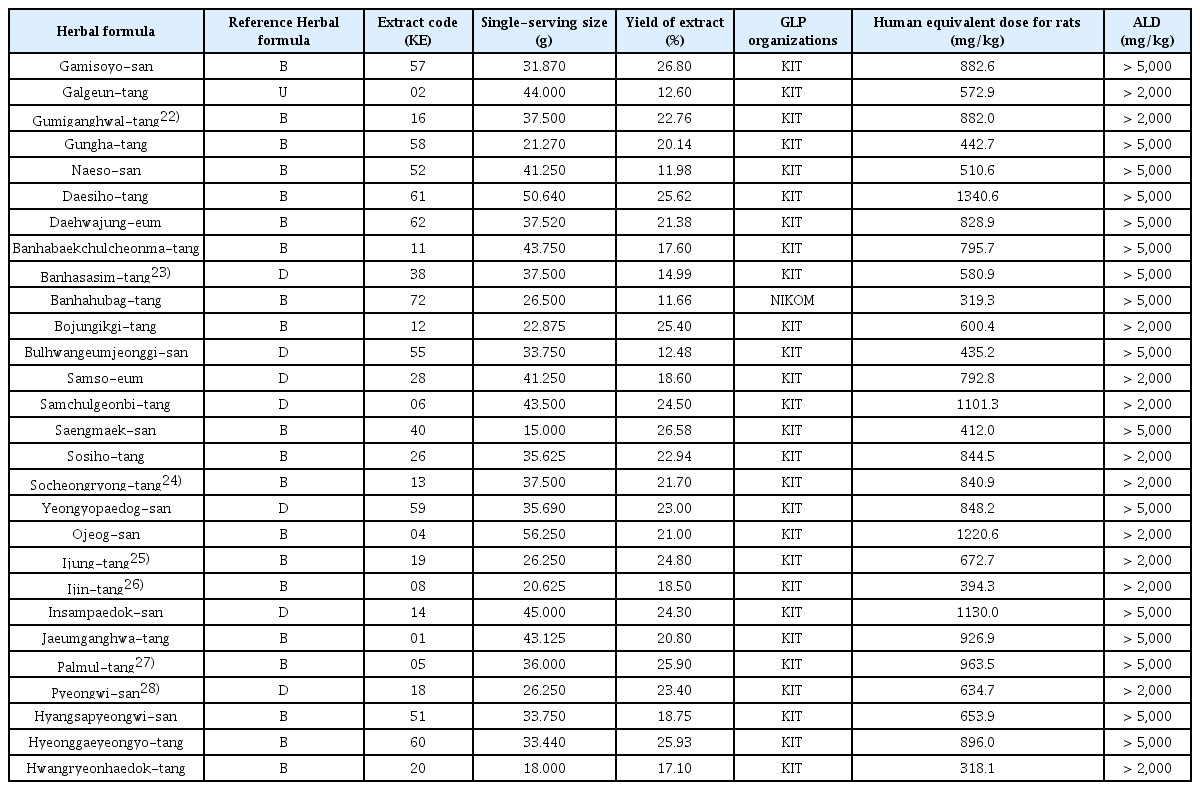
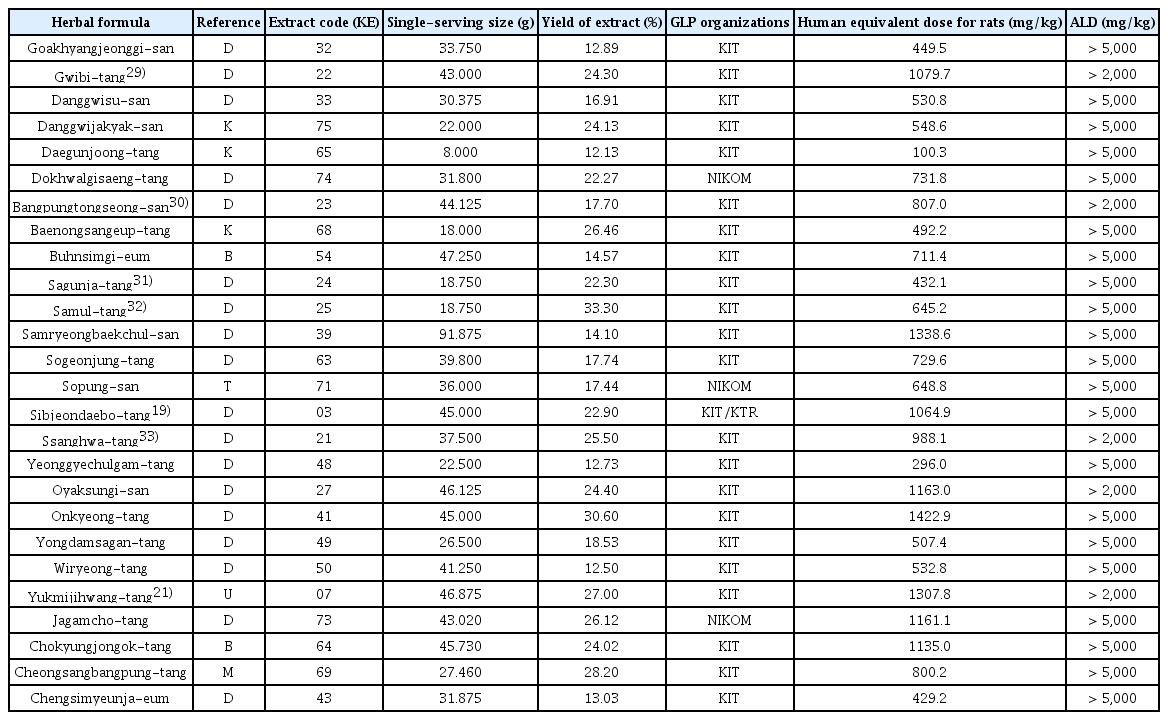
19. Ma J.Y., Huang D.S., Lee N.H., Ha H.K., Yu Y.B., Shin H.K.. 2008;Acute Toxicity Study on Sipjeondaebo-tang in Rats. Korean J. Oriental Physiology & Pathology 22(5):1192–1195.
(364)
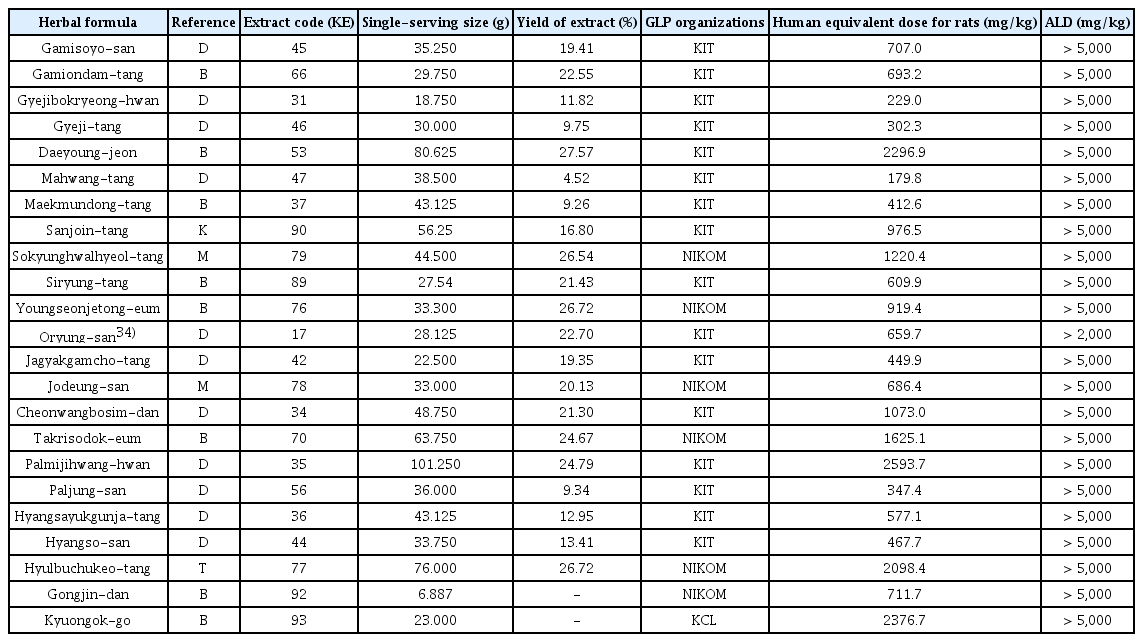
20. Ha H.K., LeeJ K., Lee H.Y., Seo C.S., Kim J.H., et al. 2010;Evaluation of safety of the herbal formula Ojeok-san: acute and sub-chronic toxicity studies in rats. J Ethnopharmacol 131(2):410–416.
https://doi.org/10.1016/j.jep.2010.07.011
.
21. Ha H.K., Lee J.K., Lee H.Y., Koh W.S., Seo C.S., Shin H.K.. 2011;Safety Evaluation of Yukmijihwang-tang: Assessment of Acute and Subchronic Toxicity in Rats. Evid Based Complement Alternat Med 2011:672136.
https://doi.org/10.1155/2011/672136
.
22. Shin I.S., Kim J.H., Ha H.K., Seo C.S., et al. 2010;Acute Toxicity Study on Gumiganghwal-tang (Jiuweiqianghuo-tang) in Sprague-Dawley Rats. The Korean Journal of Oriental Medical Prescription 18(1):79–85.
23. Yoo S.R., Jeong S.J., Lee M.Y., Shin H.K., Seo C.S., Ha H.K.. 2018;Single Oral Acute Toxicity of Banhasasim-Tang and Its Antiobesity Effect on Diet-Induced Obese Mice and 3T3-L1 Adipocytes. Evidence-Based Complementary and Alternative Medicine 2018:3865434.
https://doi.org/10.1155/2018/3865434
.
24. Lee M.Y., Seo C.S., Kim J.Y., Shin H.K.. 2015;Evaluation of a water extract of So-CheongRyong-Tang for acute toxicity and genotoxicity using in vitro and in vivo tests. BMC Complementary and Alternative Medicine 15:235.
https://doi.org/10.1186/s12906-015-0737-x
.
25. Lim H.S., Lee M.Y., Seo C.S., Shin I.S., et al. 2011;Single Oral Dose Toxicity Evaluation of Leejung-tang, a Korean Traditional Herbal Formula, in Crl:CD (SD) rats. The Journal of Korean Oriental Medicine 32(3):18–24.
26. Shin I.S., Lee M.Y., Seo C.S., Lim H.S., Ha H.K., Shin H.K.. 2012;ijin-tang, an Oriental Herbal Formula Reduces Ethanol-induced Acute Gastric Injury in Rats. J Korean Soc Appl Biol Chem (55):197–204.
https://doi.org/10.1007/s13765-012-1173-y
.
27. Ma J.Y., Huang D.S., You Y.B., Ha H.K., Shin H.K.. 2007;Acute Toxicity Study on Palmul-tang (Bawu-tang) in Mice. Kor. J. Herbology 22(2):13–16.
28. Shin I.S., Kim J.H., Ha H.K., Huang D.S., Shin H.K.. 2010;tudy on Safety of Pyungwi-san in Sprague-Dawley Rats. Journal of Physiology & Pathology in Korean Medicine 24(3):426–429.
29. Lee M.Y., Seo C.S., Kim Y.B., Shin H.K.. 2015;Safety assessment of Guibi-tang: Subchronic toxicity study in Crl:CD SD rats. Regulatory Toxicology and Pharmacology 73(2):485–493.
https://doi.org/10.1016/j.yrtph.2015.09.030
.
30. Shin I.S., Kim J.H., Ha H.K., Seo C.S., et al. 2010;Acute Toxicity Study on Bangpungtongsung-san (Fangfengtongsheng-san) in Sprague-Dawley Rats. The Journal of Korean Oriental Medical Ophthalmology & Otorhinolaryngology & Dermatology 23(1):111–117.
31. Ma J.Y., Huang D.S., You Y.B., Ha H.K., Shin H.K.. 2007;Acute Toxicity Study on Sagunja-tang (Sijunzi-tang) in ICR Mice. The Journal of Korean Medicine 28(2):200–204.
32. Ma J.Y., You Y.B., Ha H.K., Huang D.S., Shin H.K.. 2007;Acute Toxicity Study on Samul-tang (Siwu-tang) in Mice. The Korean Journal of Oriental Medical Prescription 15(2):113–117.
33. Kim S.J., Lee M.Y., Shin I.S., Seo C.S., Huh J.I., et al. 2010;ingle Dose Acute Toxicity of Ssanghwa-tang in Crl: CD (SD) Rats. Kor. J. Herbology 26(2):39–43.
34. Jeon W.Y., Lee M.Y., Shin I.S., Lim H.S., Shin H.K.. 2012;Protective Effects of the Traditional Herbal Formula Oryeongsan Water Extract on Ethanol-Induced Acute Gastric Mucosal Injury in Rats. Evidence-Based Complementary and Alternative Medicine 2012:438191.
https://doi.org/10.1155/2012/438191
.