Clinical Efficacy of herbal medicine for Chemo-Radiotherapy-induced oral mucositis
Article information
Abstract
Objectives
The purpose of this systematic review was to evaluate the effects of herbal medicine on Chemo-Radiotherapy-induced oral mucositis.
Methods
Electronic databases were used to search for studies published through 10 years until October 2022, and a randomized controlled study was conducted to evaluate efficacy of herbal medicine on chemo-radiotherapy-induced oral mucositis. Study quality was assessed using the Cochran’s risk bias tool.
Results
Two-hundred and three articles were initially searched, and 11 studies (head and neck cancer, breast cancer, colorectal cancer, esophageal cancer etc. undergoing radio-chemotherapy were included in analysis. The effect of herbal medicine on chemo-radiotherapy-induced oral mucositis, 9 studies reported that herbal medicine was more effective than the placebo group or conventional treatment. One study reported that the effect of the herbal compound treatment group was similar to that of the conventional herbal medicine, and one study reported that there was no difference in effect between the two herbal medicines and the group without treatment.
Conclusion
This study suggests that herbal medicine effectively relieves the symptoms of chemo-radiotherapy-induced oral mucositis. However, there is limited evidence that herbal medicine may relief chemo-radiotherapy-induced oral mucositis, so further investigation is needed.
Introduction
Oral mucositis (OM) is a common complication of radiotherapy, chemotherapy, a combination of radio-chemotherapy, and hematopoietic stem cell transplantation. OM is currently considered to be the most severe complication of anticancer therapy, affecting 40–80% of patients undergoing chemotherapy and almost those undergoing radiotherapy of the head and neck. OM is characterized by erythema and ulceration of the mucosal lining of the gastrointestinal tract. OM is associated with pain, difficulty in eating and swallowing, the need for enteral or parenteral nutrition, increased opioid consumption, and interruptions to cancer therapy.1–3)
As the management of OM is considered important in cancer patients, many studies have been conducted and guidelines for the management of oral mucositis have been published.1) The Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/ International Society of Oral Oncology(MASCC/ISOO) has published evidence based clinical practice guidelines for mucositis to clinical evidence based patient care and improve outcomes. The current guidelines updated in 2020 examined the evidence for the following interventions: basic oral care, anti-inflammatory agents, photobiomodulation, cryotherapy, antimicrobials, coating agents, anesthetics, analgesics, growth factors and cytokines, natural and miscellaneous agents.1)
Traditional herbal medicines are used for treating various types of mucositis. Its use has been based on the East-Asia medical theories over thousands of years. Previously studies of herbal medicine for chemo-radiotherapy-induced oral mucositis (CROM) have been conducted on case report4), and mechanism of action of TJ-14(Hangshashinto)5–7), and Oncheongeum8) for oral mucositis, and clinical studies on herbal medicine gargles9,10) have been conducted. Despite the progress of research on various drugs and management, an effectiveness of treatment has not been established. Therefore, the aim of this systematic review is to evaluate the effectiveness and safety of herb medicines or herbs treatment in relieving CROM.
Methods
1. Data Sources and Search Strategy
The following electronic databases were searched to identify relevant studies published through 10 years until September 2022: Pubmed, MEDLINE, the Cochrane Library (CENTRAL), Research Information Sharing Service (RISS), Chinese Medical Database (CNKI). The search terms used were as follows: (neoplasm OR cancer OR tumor Or malignant OR chemotherapy OR radiotherapy) AND (herbal medicine OR traditional chinese medicine OR decoction OR herb) AND (oral mucositis OR stomatitis OR 口 炎 OR 口腔黏膜炎) in Korean, English, and Chinese. The databases used in this study were independently searched manually.
2. Study Selection
After reviewing the titles and abstracts of the searched papers, the first selection was made, and then the papers to be used for systematic review were selected through the original text review. The criteria for papers selection included the following criteria. All randomized controlled trials (RCTs) were included. Observational, cohort, case-control, case series, qualitative, laboratory studies, and uncontrolled trials were excluded. CROM was diagnosed using published diagnostic criteria. No restrictions were placed on race, age, or sex. The interventions selected involved herbal medicine of and ingredient to with no limit on the total dosage and treatment session and included all types of control intervention, including placebo and conventional medication.
3. Data Extraction and Risk-of-Bias Assessment
Two authors (SWP and MJK) performed the data extraction and collated information independently on sample size, age range, interventions, treatment durations, outcomes, and results. Any disagreement between the two authors was resolved by discussion and decided to seek the opinion of a third researcher (SWL) along with consideration. Risk of bias was assessed using the following seven criteria from Cochrane Collaboration’s risk of bias tool.
Results
1. Study selection
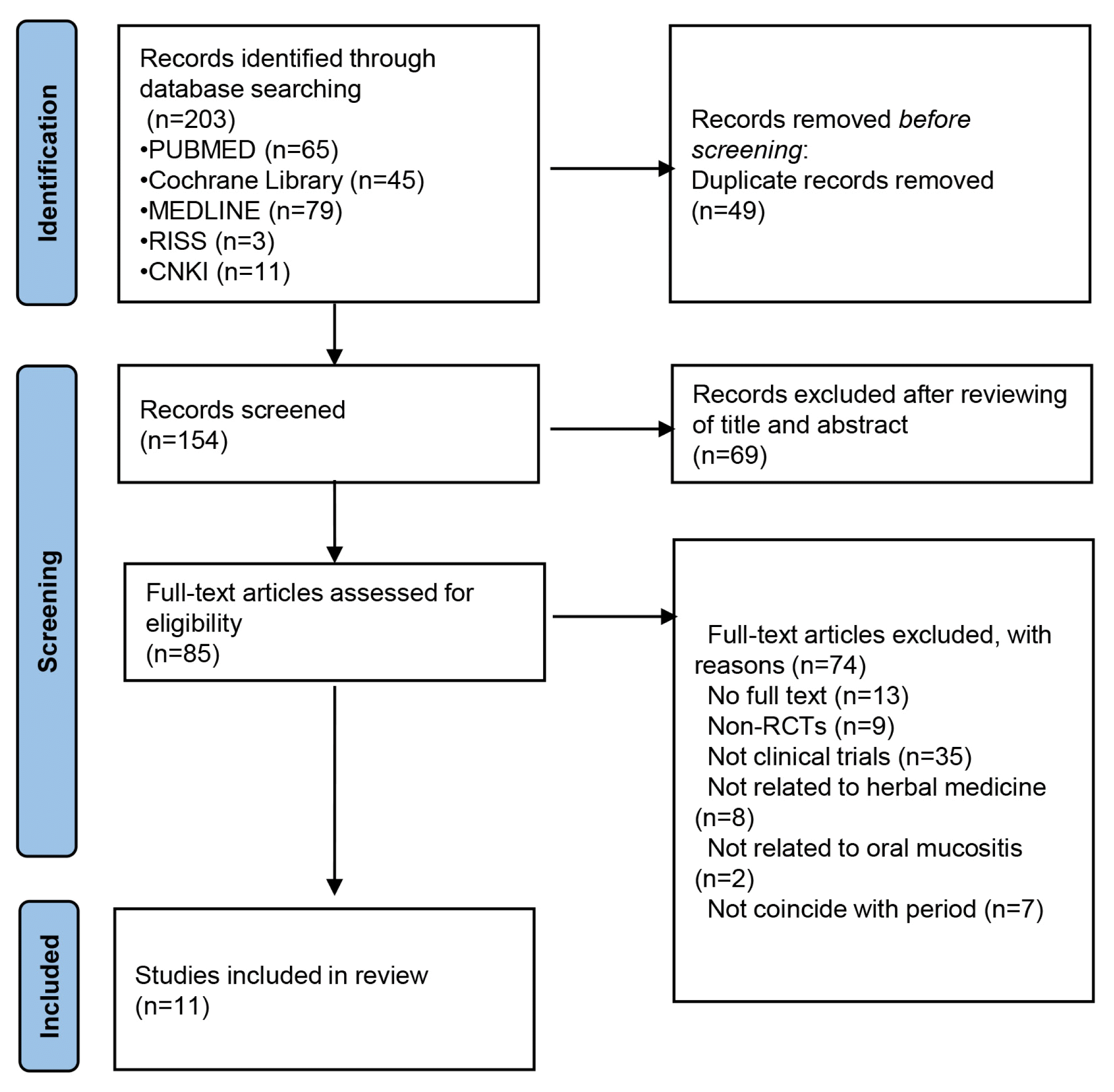
A total of 203 studies were originally identified by searching 5 databases using search terms and search strategies. Of these, 49 were excluded for duplication. The remaining 154 studies were reviewed with a focus on titles and abstracts, and 69 were primarily excluded. Subsequently, another 74 studies were excluded for the following reasons: 13 articles could not find the full text, 9 were not randomized, 35 did not include a clinical trial, 8 did not use Herbal medicine, 2 did not discuss CROM, and 7 did not coincidence period. Finally, 11 studies11–21) were selected, and the flow chart of the selection process is provided in Figure 1.
2. Description of the Included Trials
The meta-analysis was not possible due to the large heterogeneity in the study, such as different intervention methods and different evaluation indicators. Therefore, the research design, classification of disease, applied intervention, control group, evaluation index, and adverse effects of the selected papers were arranged for each paper for descriptive analysis, and the results were summarized in a Table 1, 2.
1) Characteristics of included studies
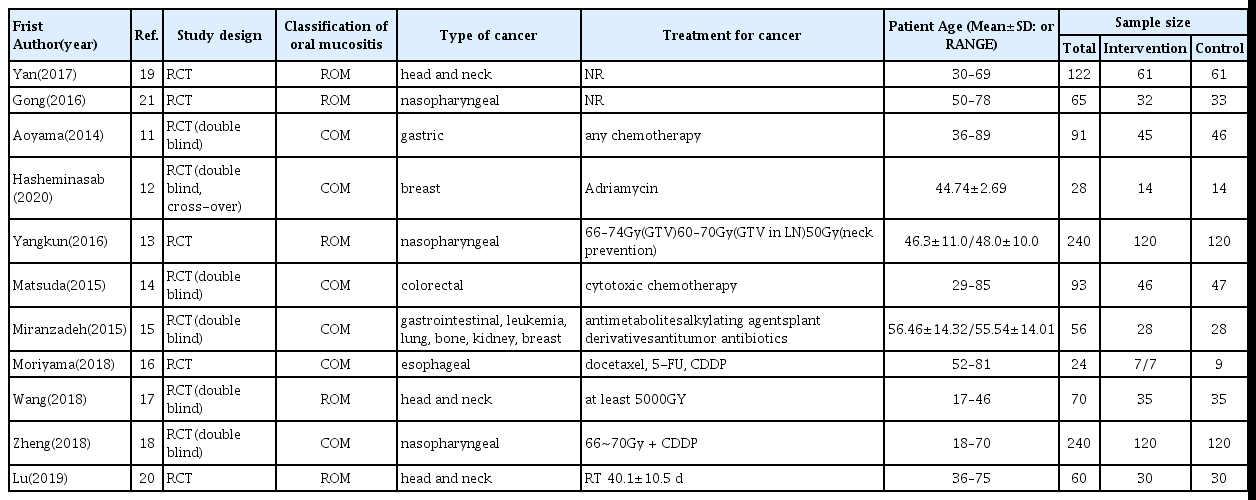
Characteristic data from the 11 studies11–21) are summarized in Table 1. Sample sizes ranged from 24 to 240, and patients’age from 18 to 90 years. Therapy durations ranged from 2 weeks to 3 months during radiotherapy or chemotherapy. All 11 studies11–21) reported type of cancer. Of these, 6 trials13,17–21) were studies of head and neck cancer. And the 4 studies11,12,14,16) conducted a study on colorectal cancer, esophageal cancer, gastric cancer, and breast cancer, 1 study15) was all included gastrointestinal cancer, leukemia, lung cancer, bone cancer, kidney cancer, and breast cancer. 9 studies11–18,20) reported types of radio-chemotherapy during the treatment of carcinoma, but 2 studies19,21) did not. 5 cases13,17,19–21) conducted radiation therapy alone to treat for head and neck cancer. In 5 studies11, 12,14–16) were performed only chemotherapy, and it was a cytotoxic chemotherapy agent, the standard treatment for each cancer type. 1 study18) was conducted chemo-radiotherapy.
2) Characteristics of herbal medicine intervention
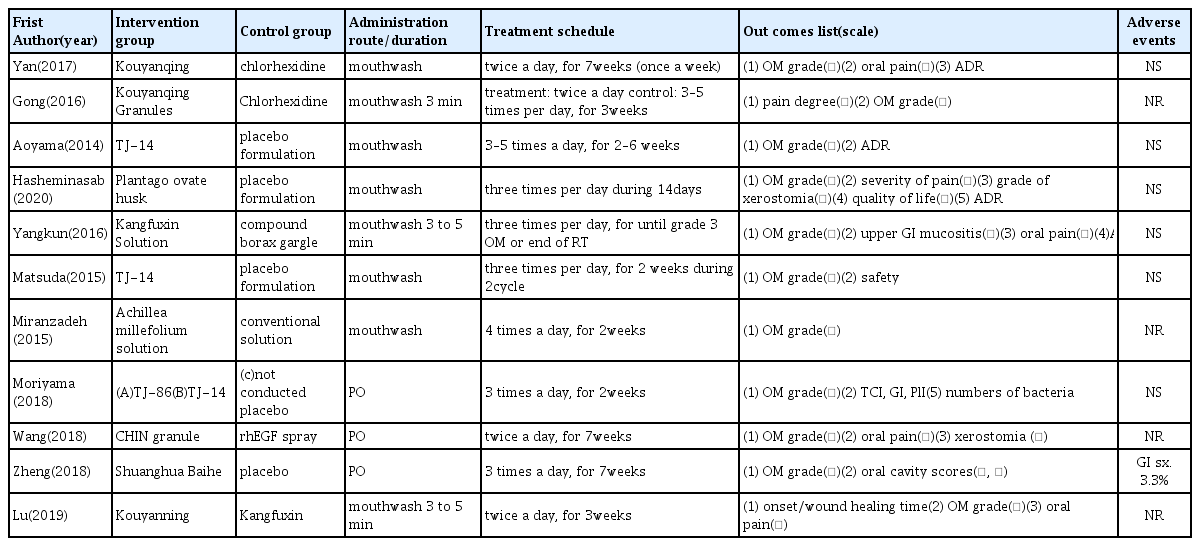
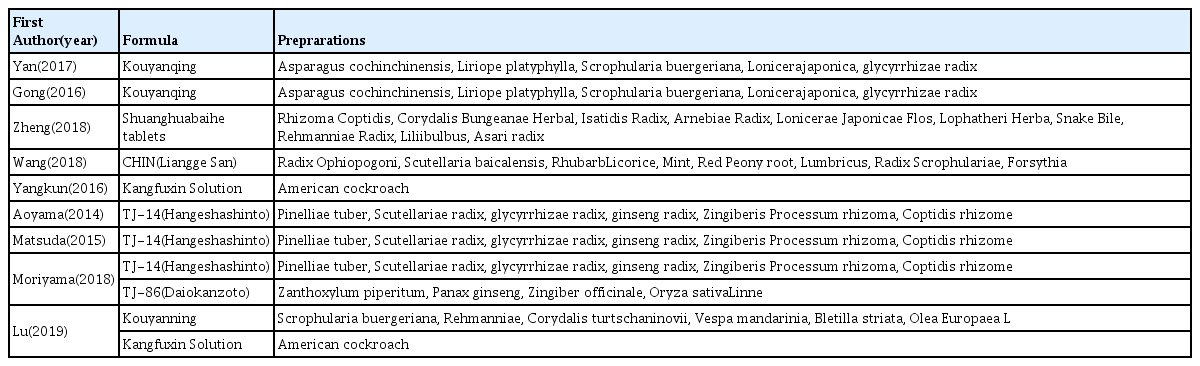
Of the 11 articles11–21) analyzed for the therapeutic effect, 3 articles16–18) used oral administration herbal medicines, the formulations were decoction; CHIN granule (modified Liangge San), sherbet form; Daiokanzoto, Hangeshashinto and tablet; Shuanghua baihe. 8 studies11–15, 19–21) used gargle solution; Kouyangqing, TJ-14, Plantago ovate husk suspension, Achillea millefolium solution, Kangfuxin, Kouyanning were used in the treatment group. 4 studies11,12,14,18) have compared herbal therapy with placebo, 5 studies13,15,17,19,21) have compared herbal therapy with conventional treatment; chlorhexidine gargle, compound borax gargle, lidocaine, dexamethasone, sucralfate, rhEGF spray. one study16) compared herbal medicine to the untreated control group, and one study20) have evaluated the efficacy of between herbal medicine. Materials used in treatment from the 11 studies are summarized in Table 3.
3) Outcome measure
All the studies11–21) examined in this study used the oral mucositis grade such as radiation therapy oncology group(RTOG) criteria, World Health Organization(WHO) grade, Common Terminology Criteria for Adverse Events version(CTCAE) criteria. 5 studies12,13,17,19, 20) used visual analog scale(VAS) or number rating scale(NRS) for calculating oral pain. In addition, grade of xerostomia, quality of life, adverse drug reaction (ADR) were reported as an effect.
The effect of herbal medicine on CROM, 911–15, 17–19,21) of 11 studies reported that herbal medicine was more effective than the placebo group or conventional treatment. No effectiveness was reported in Daiokanzoto and Hangeshashinto16) compared with control group. The other one study20) was conducted comparing the effects between herbal medicines; Kouyanning, Kangfuxin, and reported no difference in clinical effects on OM, therefore both medicines are considered to use to relieve the symptoms of OM. Herbal medicines suggested in studies that have been effective included compound herbal medicine; Kouyangqing, Kangfuxin, TJ-14(Hangeshashinto), CHIN granule(modified Liangge San), Shuanghua Baihe tablets, single herbal medicine; Plantago ovate husk, Achillea millefolium. In particular, traditional herbal medicine TJ-14(Hangeshashinto) was effective in grade 1 and 2 OM, and there was no effect in severe OM grade 3. The duration of treatment reported in the selected studies ranged from 2 weeks to 3 months depending on the schedule of chemotherapy or radiotherapy.
7 studies11–14,16,18,19) reported adverse effects during the treatment. Of these, 6 studies11–14,16,19) reported no significant adverse effects. In one study18), GI adverse events 3.3% were reported in Shuanghua Baihe tablets group.
3. Risk of Bias
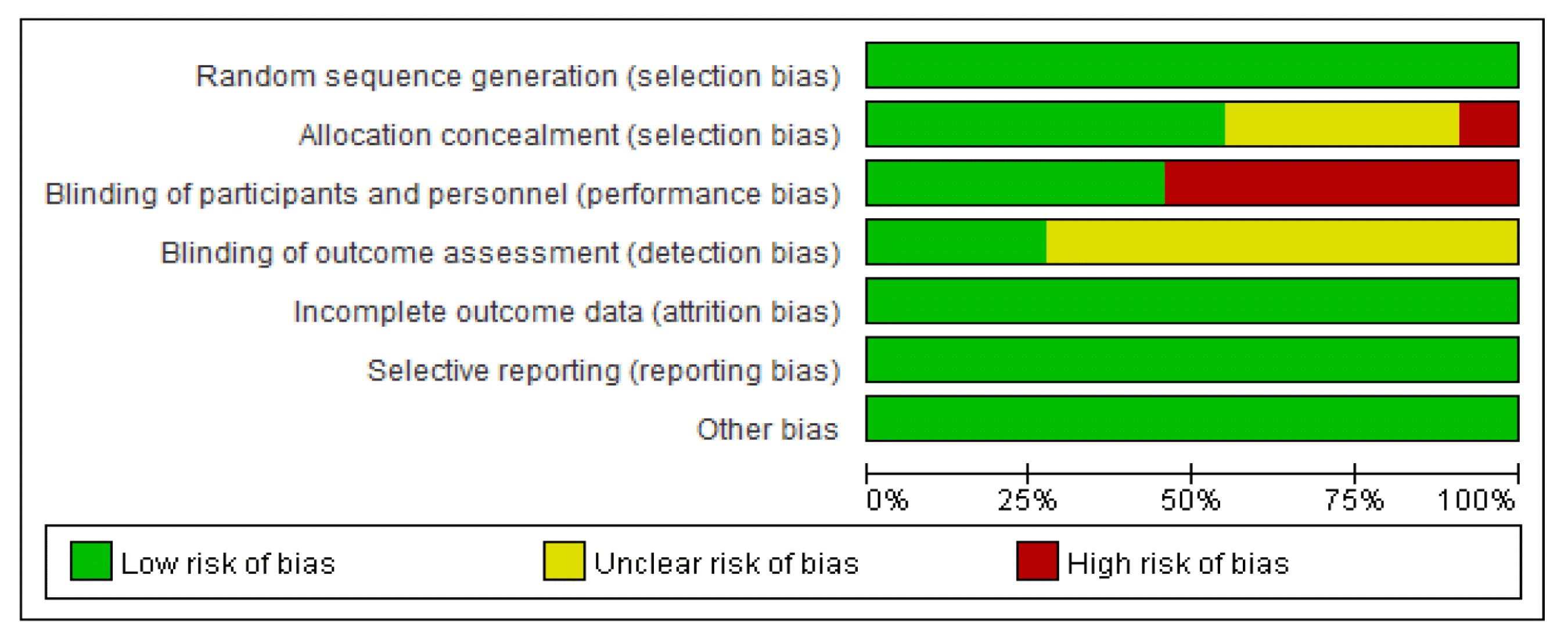
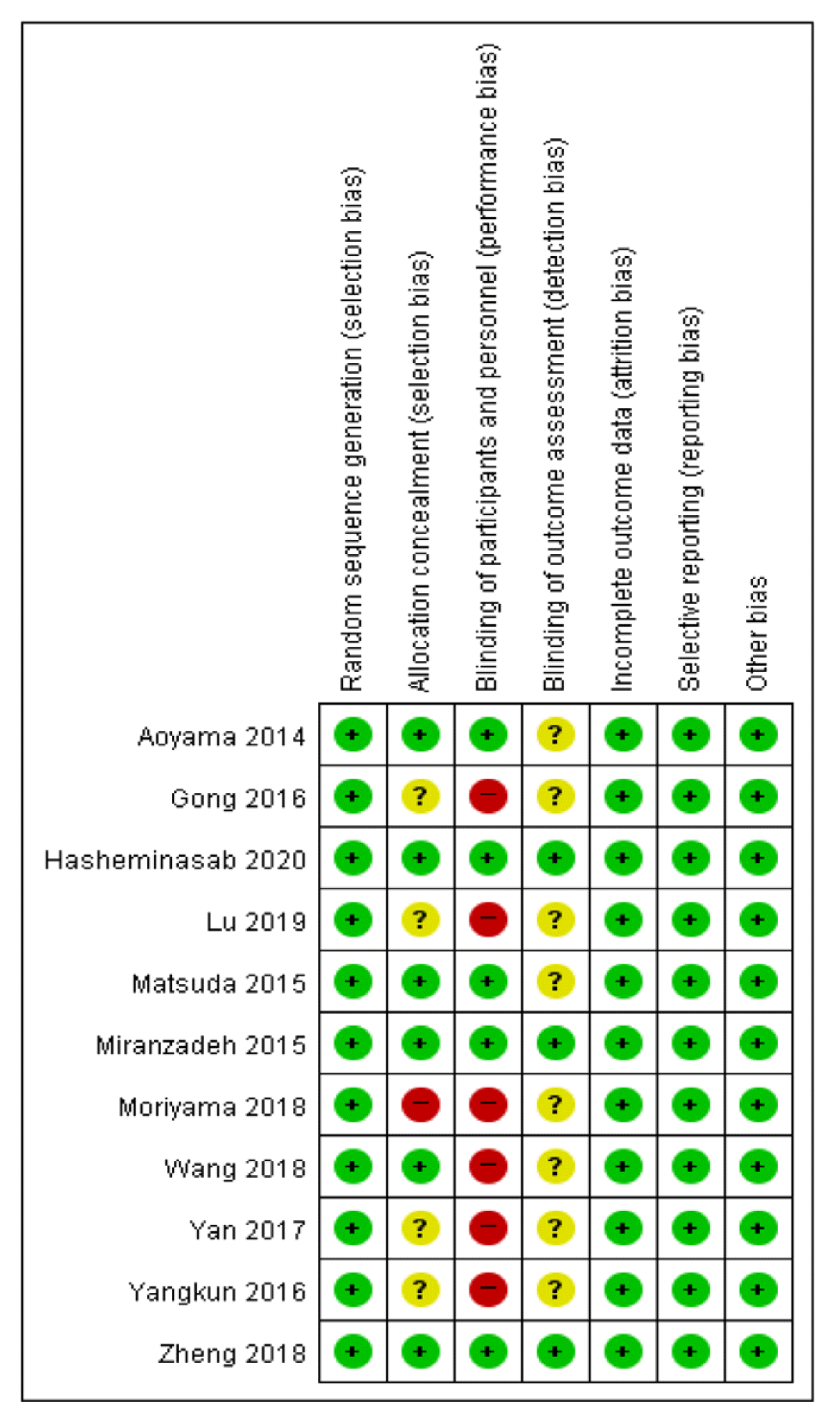
All studies11–21) were described as ‘randomized’ and reported the methods of random sequence generation such as using computer program, random number table. 6 studies11,12,14,15,17,18) described allocation concealments, 1 study16) had high risks of bias for allocation due to divide randomly into three groups using the envelop method without a blind. 6 studies13,16,17,19–21) had high risks of bias for participant and personnel blinding, and 5 studies11,12,14,15,18) had low risk. 3 trials12,15,18) used outcome assessment blinding. All studies reported complete outcome data, and did not reported selective data. The reviewers had no disagreements the risk of bias. (Figure 2, 3)
Discussion
This systematic review analyzed 11 randomized trials with 1089 total participants to reveal that herbal medicines can improve symptoms of CROM. As for the overall clinical efficacy; OM grade, oral pain, our analysis revealed that experiment groups showed better efficacy than control groups without severe adverse effects. Recently published SR28–31) of OM that occurred after radiation and chemotherapy, the clinical efficacy of traditional Chinese therapy(Yin-Yang) and the herbal medicine gargle was analyzed, and the studies was conducted until 2021. In addition, there are studies analyzing the effects of herbal medicine treatment on xerostomia31) caused by radiation and chemotherapy. These are well-designed SRs, and the effects of herbal medicine also yielded positive results. This study analyzed and reviewed studies on OM that occurred after radiation and chemotherapy for 10 years until 2022.
OM is one of the most debilitating morbidities after radio-chemo therapy.22) In order to accurately identify the symptoms of OM, objective assessment and subjective assessment. Mucositis assessment tools include the Common Terminology Criteria for Adverse Events version (CTCAE), Oral Assessment Guide (OAG), Oral Mucositis Daily Questionnaire (OMDQ), and the World Health Organization (WHO) Oral Toxicity Assessment Tool.23) Chemotherapy and radiotherapy have direct effect on epithelial cells leading to epithelial thinning and loss of barrier and oxidative stress followed by production of inflammatory cytokines (Tumor necrosis factor TNF-α, Interleukin IL-1,6) has been established as the major causative factor of OM.24,25) In addition, the development of OM is associated with the type of anticancer therapy, dosage, and how to deliver it.26) Kouyanqing granules have the functions of detoxification and swelling. Shuanghuabaihe tablets, TJ-14 contained Berberine, the main active component of the Coptidis Rhizoma, and has been demonstrated to possess antifungal, antibacterial, antioxidant, anti-inflammatory properties.11,14,27) Modified LianggeSan, in a recent clinical study, combined LianggeSan and conventional medicine showed a significant effect on mucositis patients and reduced the relapse rate, and its mechanism may be to inhibit the release of inflammatory mediators by promoting the secretion of anti-inflammatory mediators, regulating the balance between inflammation and anti-inflammatory response. Also, A. millefolium has therapeutic antibacterial effect, antispasmodic and anti-inflammatory effects, P. ovata seeds is useful to treat oral cavity inflammation, burning sensation of the tongue and mouth dryness by anti-inflammatory, antioxidant, antibacterial activities. Kangfuxin may reduce the pain and discomfort of patients after chemo-radiotherapy by inhibiting radiation damage-induced opening of the calcium-dependent potassium channel. Most of the herbal medicines in the study have effects; anti-inflammatory and antioxidant effects, and these effects resulted in effective treatment of CROM.
This study was comprehensive review to assess the effectiveness of herbal medicines on CROM. A systematic review was conducted by selecting the 11 randomized controlled clinical studies related to the topic among the papers published over 10 years by using a search term online databases. There are several limitations of this systematic review regarding the use of herbal medicine for the treatment of CROM that should be considered further. The small sample sizes and low, questionable methodological qualities, duration of the intervention, and OM scaling makes it conclusion of lack of evidence. The significance of this study is that healing and symptomatic care of OM can be performed without severe side effects. And herbal medicine used in this study may be additional options for patients who do not respond to conventional treatment. In addition, further studies should follow using consistent assessment, long duration of intervention, large-scale clinical study to draw a more definitive conclusion.
Conclusions
This study suggests that some herbal medicine effectively relieves the symptoms of CROM. However, there is limited evidence that herbal medicine may relief CROM, so further investigation is needed.