The effect of isoflavonoid contents in SH003 and its subfractions on breast cancer
Article information
Abstract
Objectives
We investigated the isoflavone contained in SH003 and its fractions, and the effect of these components on the inhibition of breast cancer.
Methods
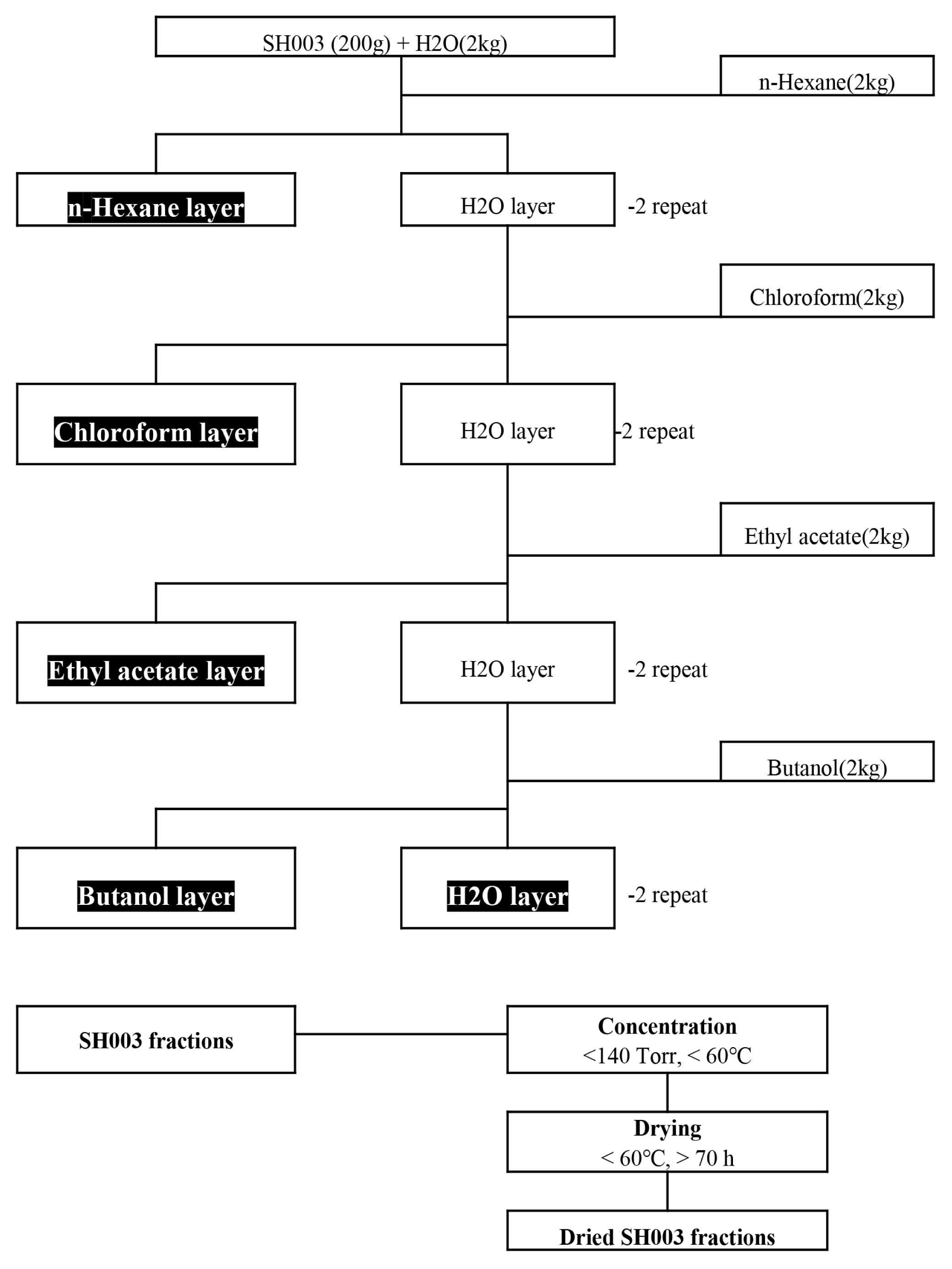
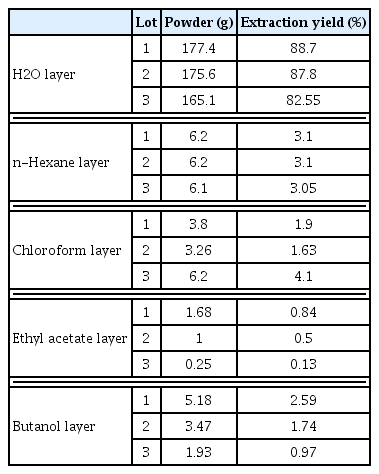
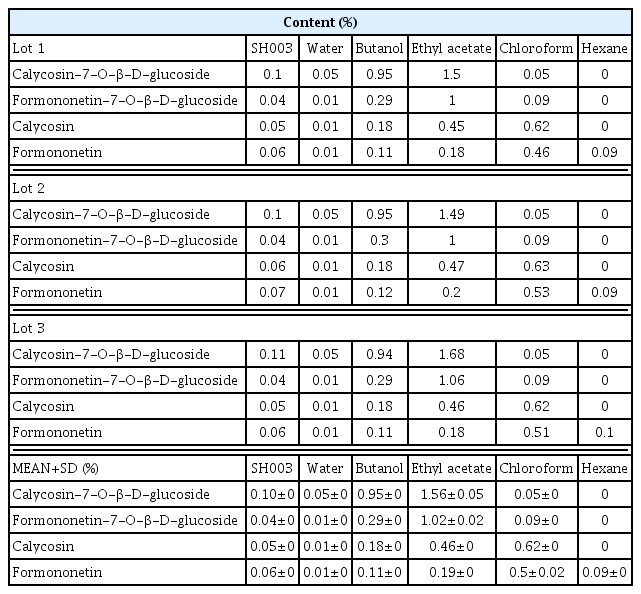
The isoflavones in solvent fractions of SH003 extract were identified by UPLC-MS and its contents were quantified using HPLC analysis. The estrogenic activity of SH003 or fractions was assessed by ERE luciferase assay in estrogen receptor (ER)-positive MCF-7 cells. To test the breast cancer inhibitory effect, the cell viability was measured using an MTT assay.
Results
In this study, we demonstrated that SH003 and fractions contain 4 isoflavones which are calycosin-7-β -D-glucoside, formononetin-7-β-D-glucoside, calycosin, and formononetin. Despite containing isoflavones, estrogen-dependent transcription activity was not altered by both SH003 and fractions. On the other hand, SH003 and fractions inhibited the cell viability of breast cancer. In addition, its isoflavone components also showed reduced cell viability in various breast cancer cells.
Conclusions
Overall, the phytoestrogen included in SH003 and fractions did not influence the estrogenic activity, emphasizing the safety of SH003 and fractions in breast cancer treatment.
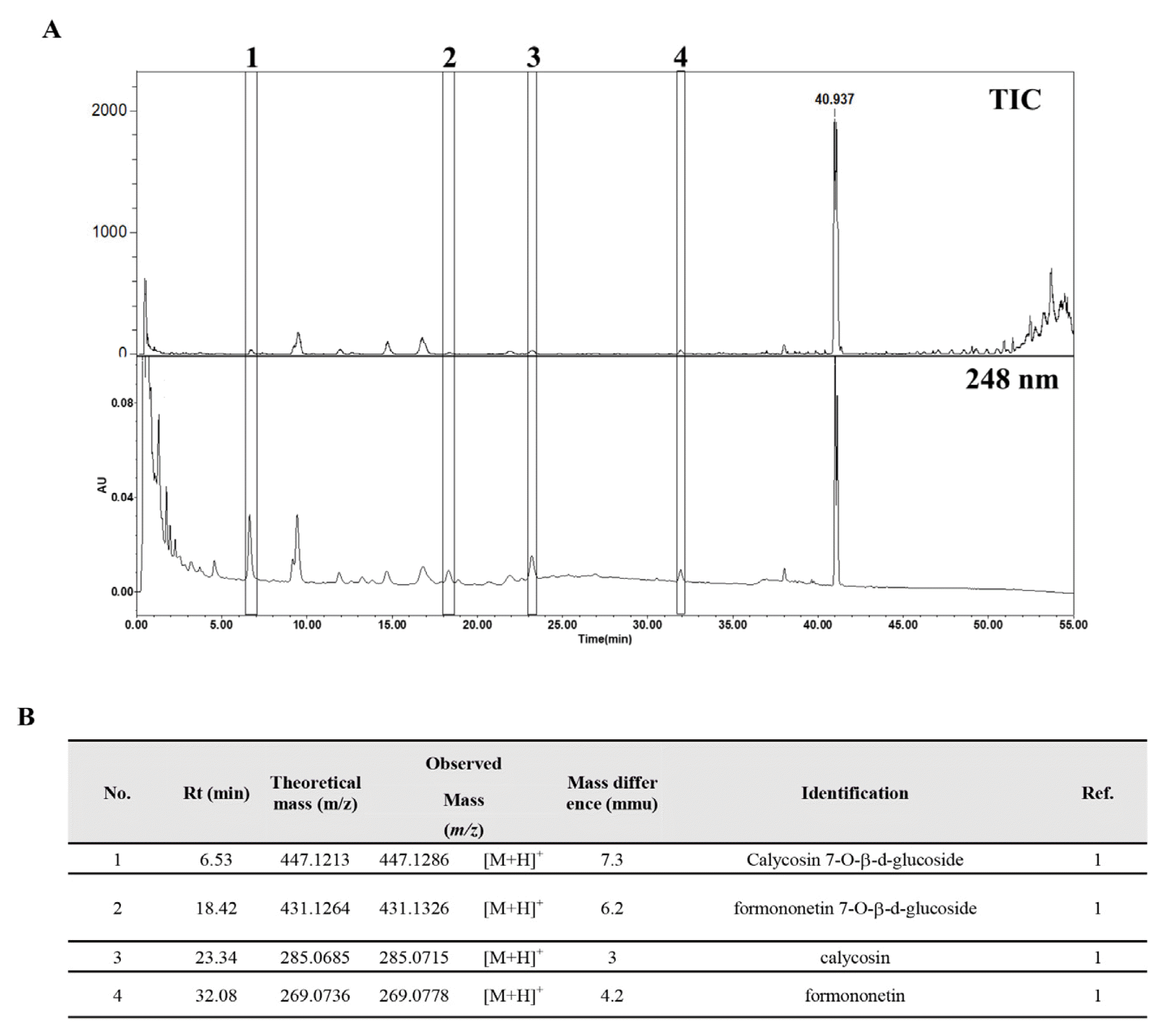
UPLC-MS chromatograms of SH003
(A) UPLC-MS TIC (top) and UPLC-UV (bottom) analysis. (B) The identification of major isoflavones. 1: calycosin 7-O-β-D-glucoside, 2: formononetin 7-O-β-D-glucoside, 3: calycosin, 4: formononetin.
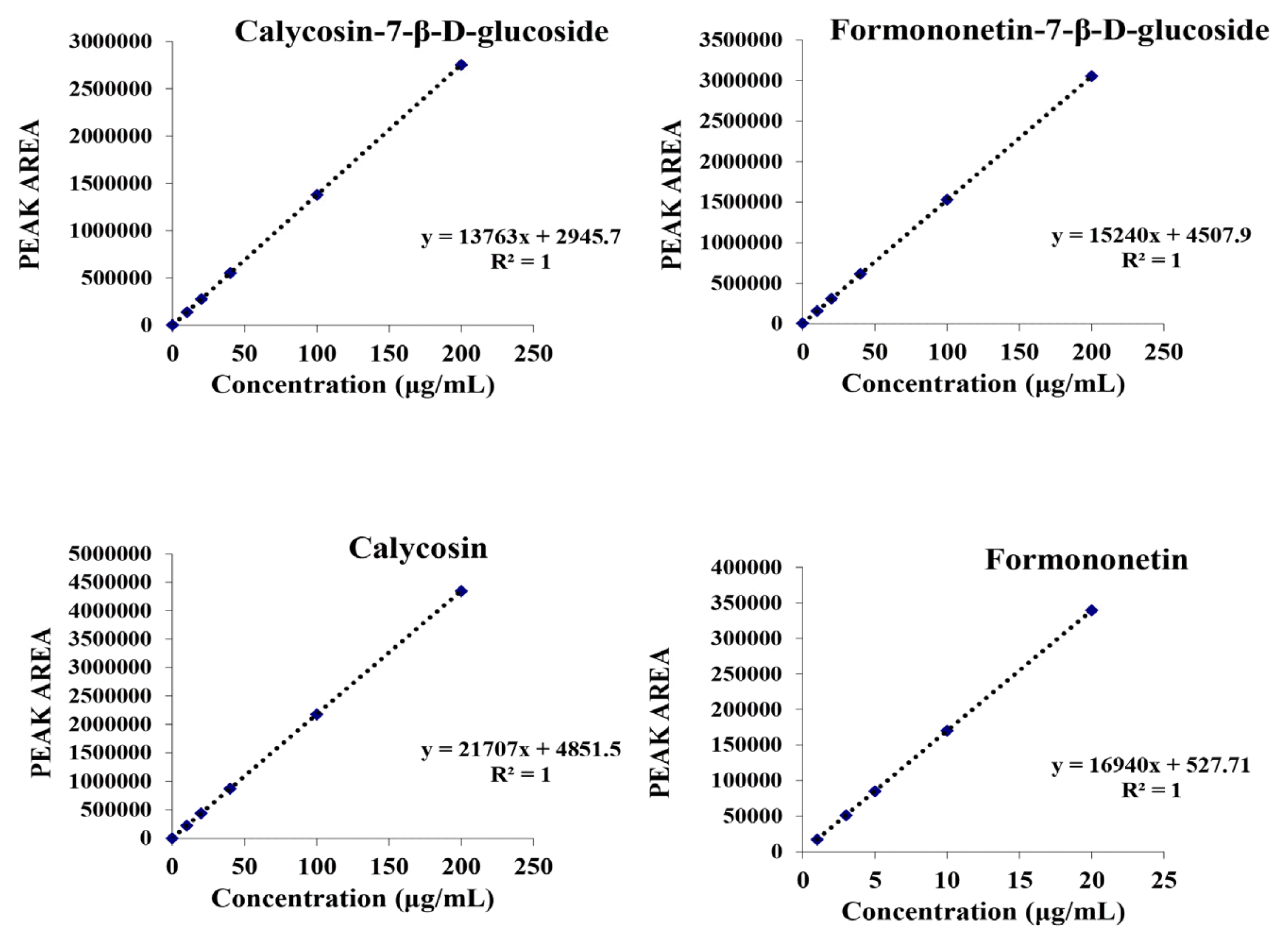
HPLC Standard curve of isoflavone
The standard curve was determined by peak areas calculated following the concentration (μg/mL) of the injected isoflavone standards.
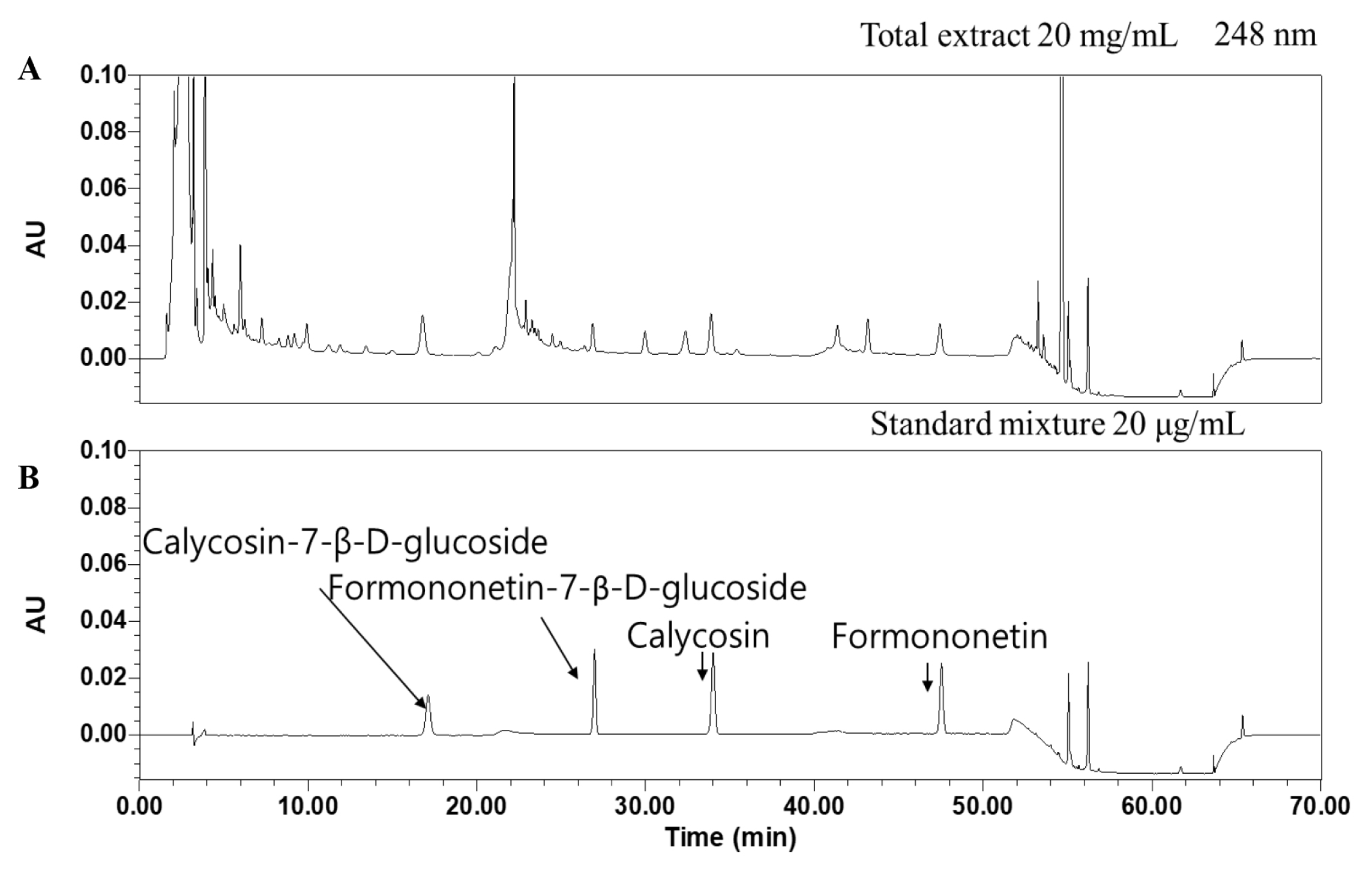
HPLC chromatograms of isoflavone standards and SH003 crude extract
(A) SH003 extract. (B) Isoflavone standards.
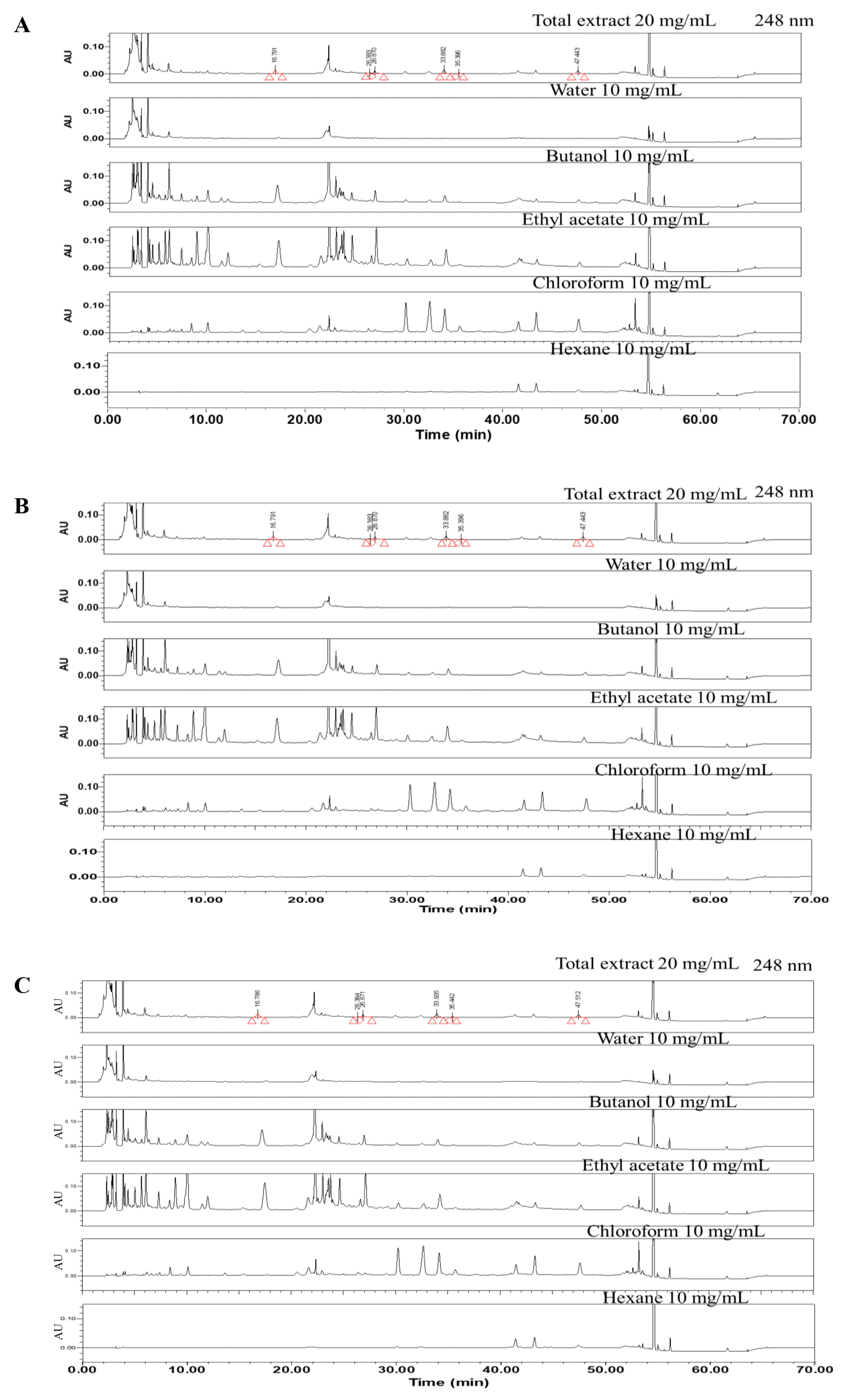
A comparison of HPLC chromatograms between the batch number of SH003 extract
(A) SH003 Lot1. (B) SH003 Lot2. (C) SH003 Lot3.
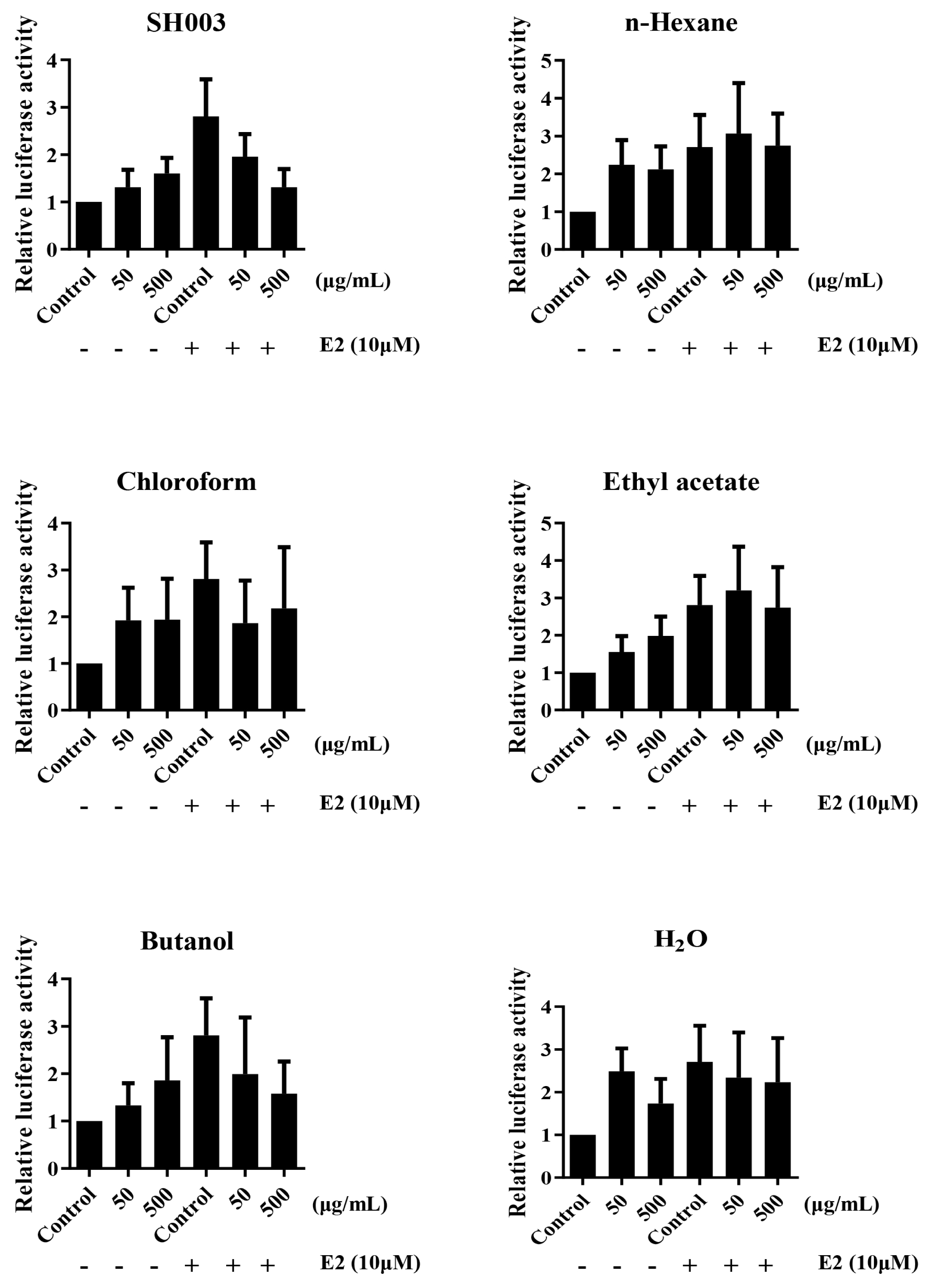
The analysis of ERE luciferase activity by SH003 and fractions
MCF-7 cells were transfected with ERE reporter plasmid for 24 h. Transfected cells were treated with SH003, fractions, and E2 for 24 h. Luciferase activity was measured using Fluoroskan FL Microplate Luminometer (ThermoScientific, USA). The Renilla luciferase is used to normalization.
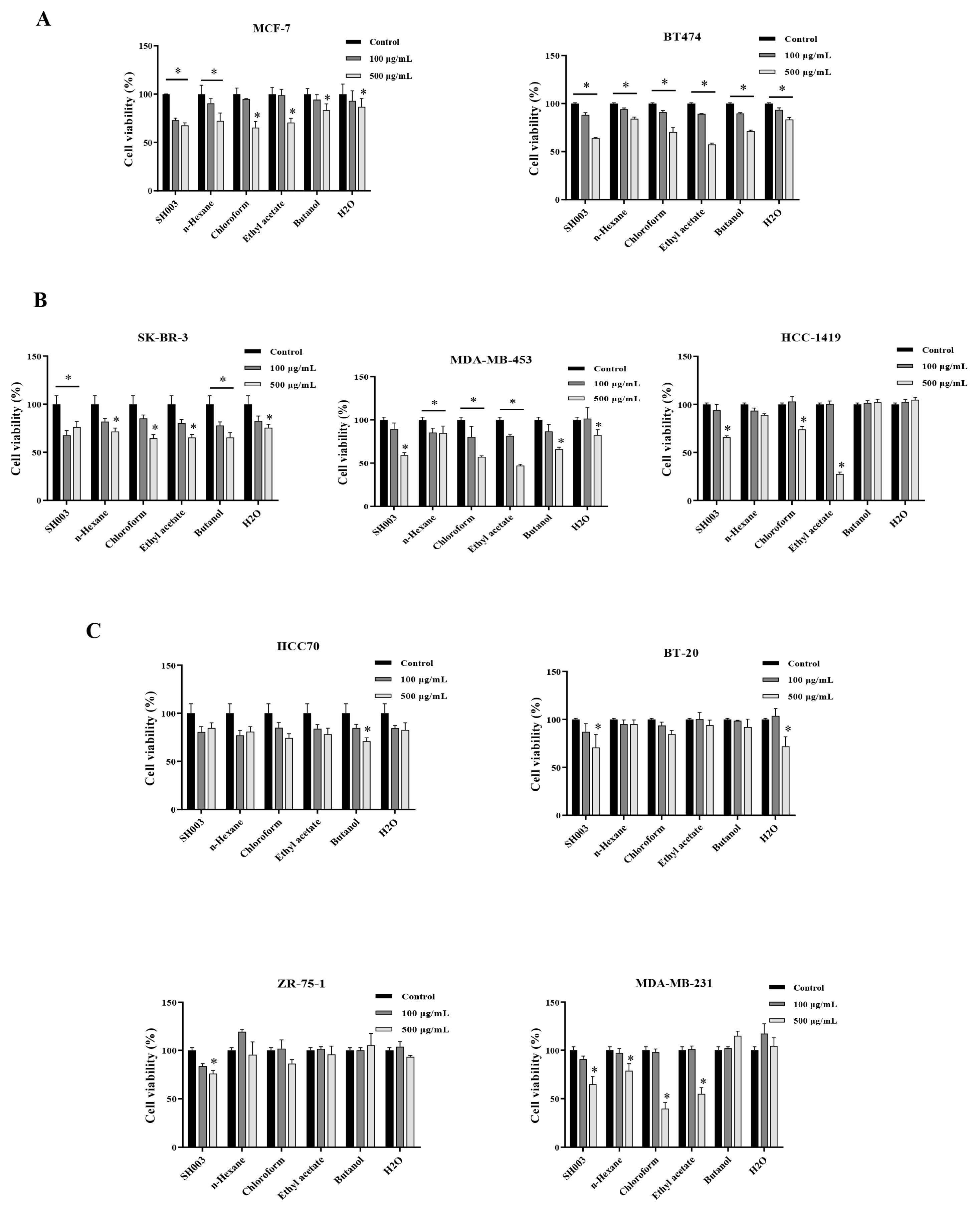
The effect of SH003 and fractions on breast cancer cell death
Breast cancer cells were treated with SH003 and fractions for 48h at indicated dose. The cell viability was detected by MTT assay. Data represent the mean ±SD. * P < 0.05. (A) ER+ breast cancer, (B) HER2+ breast cancer, (C) Triple-negative breast cancer (TNBC).
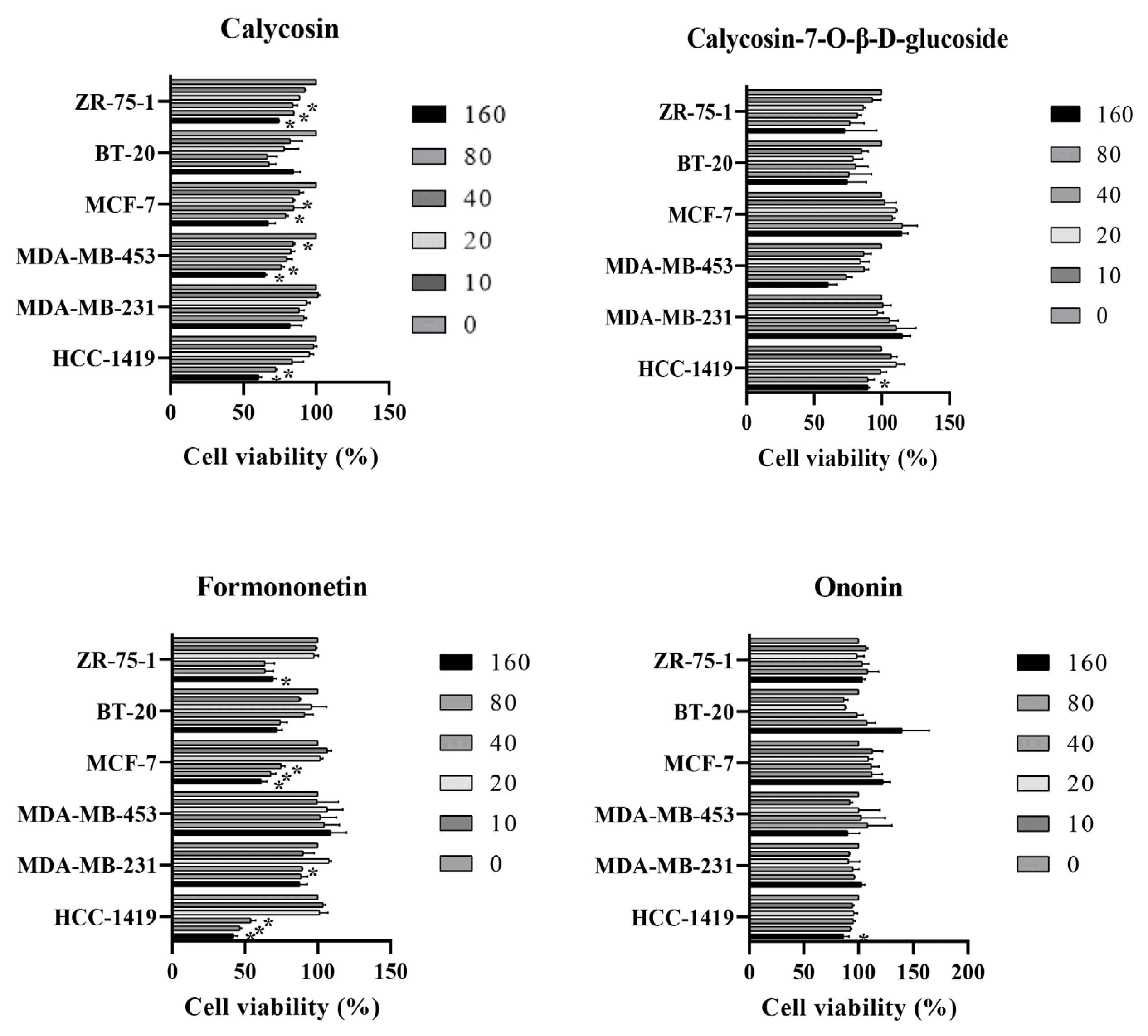
The effect of isoflavone components on breast cancer cell death
Breast cancer cells were treated with 4 isoflavone for 48 h. The cell viability was measured by MTT assay. * P < 0.05.
Acknowledgement
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A5A2019413).