Two Cases of Korean Medicine Treatment for Patients Complaining of Long-lasting Discomfort after COVID-19 Vaccination
Article information
Abstract
Objectives
This study examined the effectiveness of Korean medical treatment for two patients complaining of discomfort after receiving Pfizer COVID-19 vaccine.
Methods
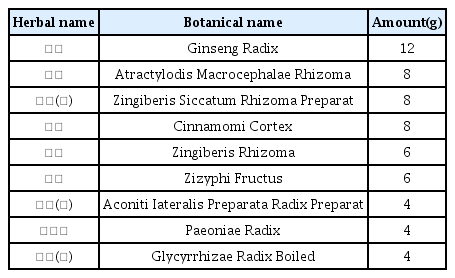
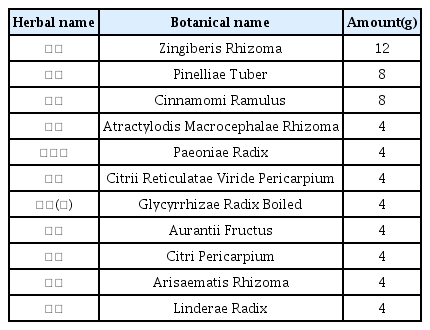
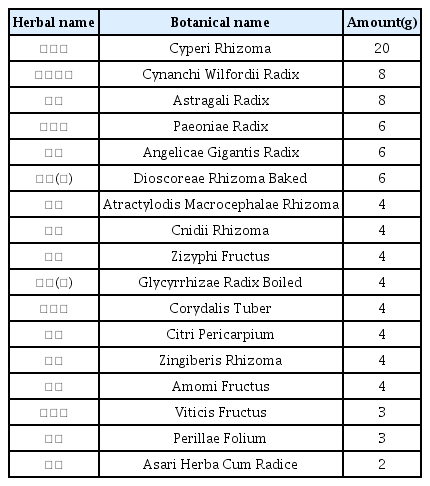
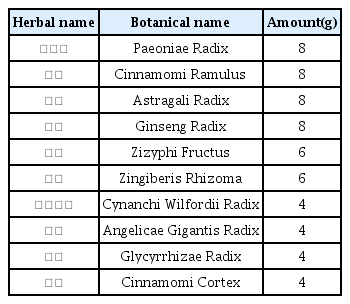
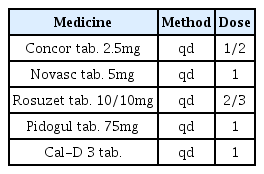
The patients were hospitalized for 50 days and 12 days, respectively. They were treated with herbal medicine, acupuncture, electroacupuncture, and moxibustion. We used the Numerical Rating Scale (NRS) on numbness in extremities and headache, Manual Muscle Testing Grading System (MMT), Criteria for Sweating Categorization, and 36-Item Short Form Health Survey (SF-36) to evaluate the clinical effects of the treatment.
Results
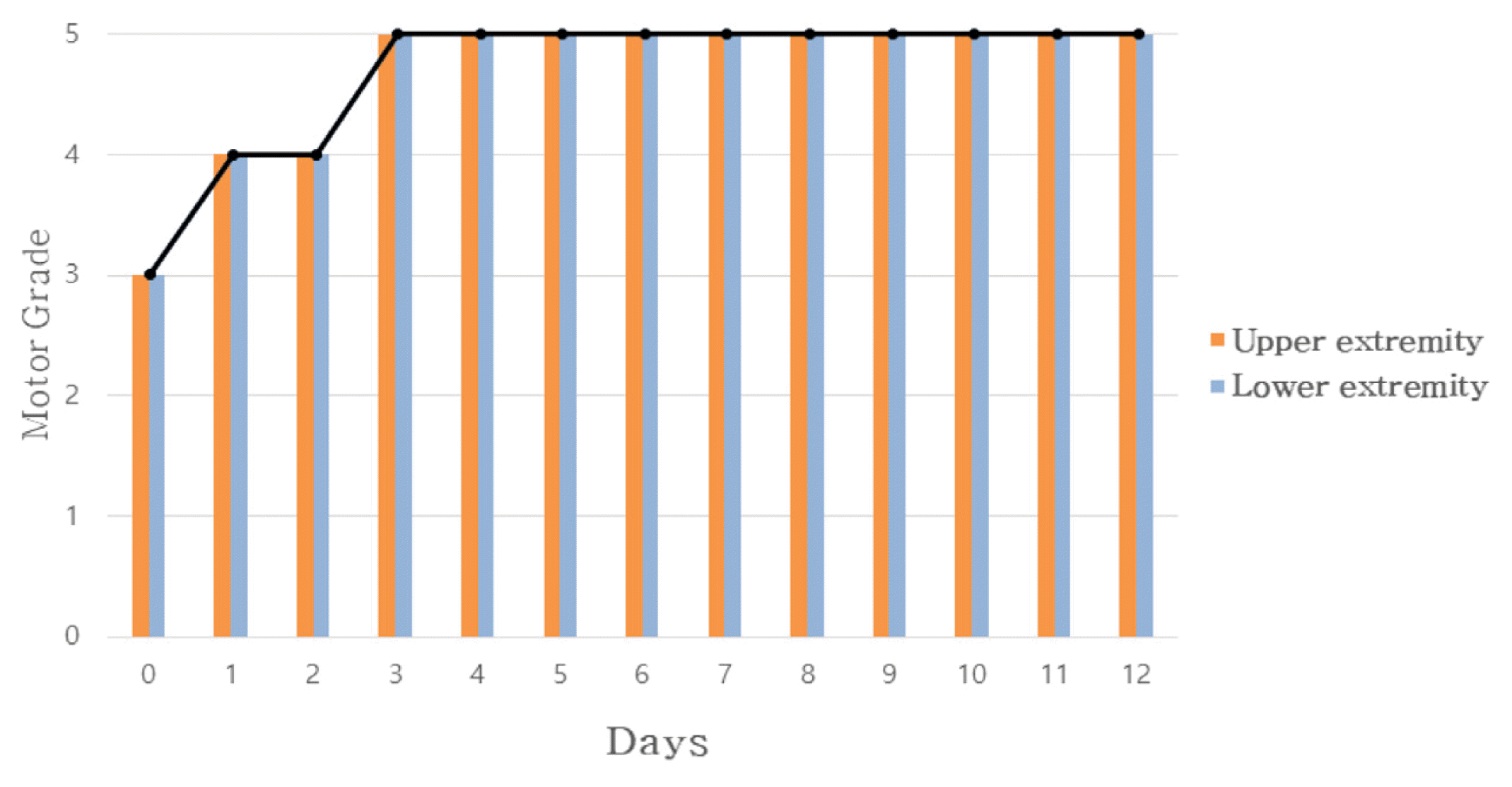
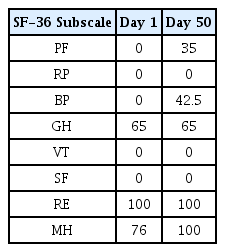
In Case 1, headache improved from peak NRS 9 and average NRS 7 on admission day to both NRS 3 on discharge. The SF-36 score was also increased, suggesting that the quality of life was improved. In Case 2, numbness in the extremities improved from NRS 8 on the day before admission to NRS 2 on discharge, and general condition also improved.
Conclusions
This study suggests that Korean medicine can be an effective treatment for patients who experience long-lasting discomfort after being vaccinated with COVID-19, but with no abnormal findings in the examination.
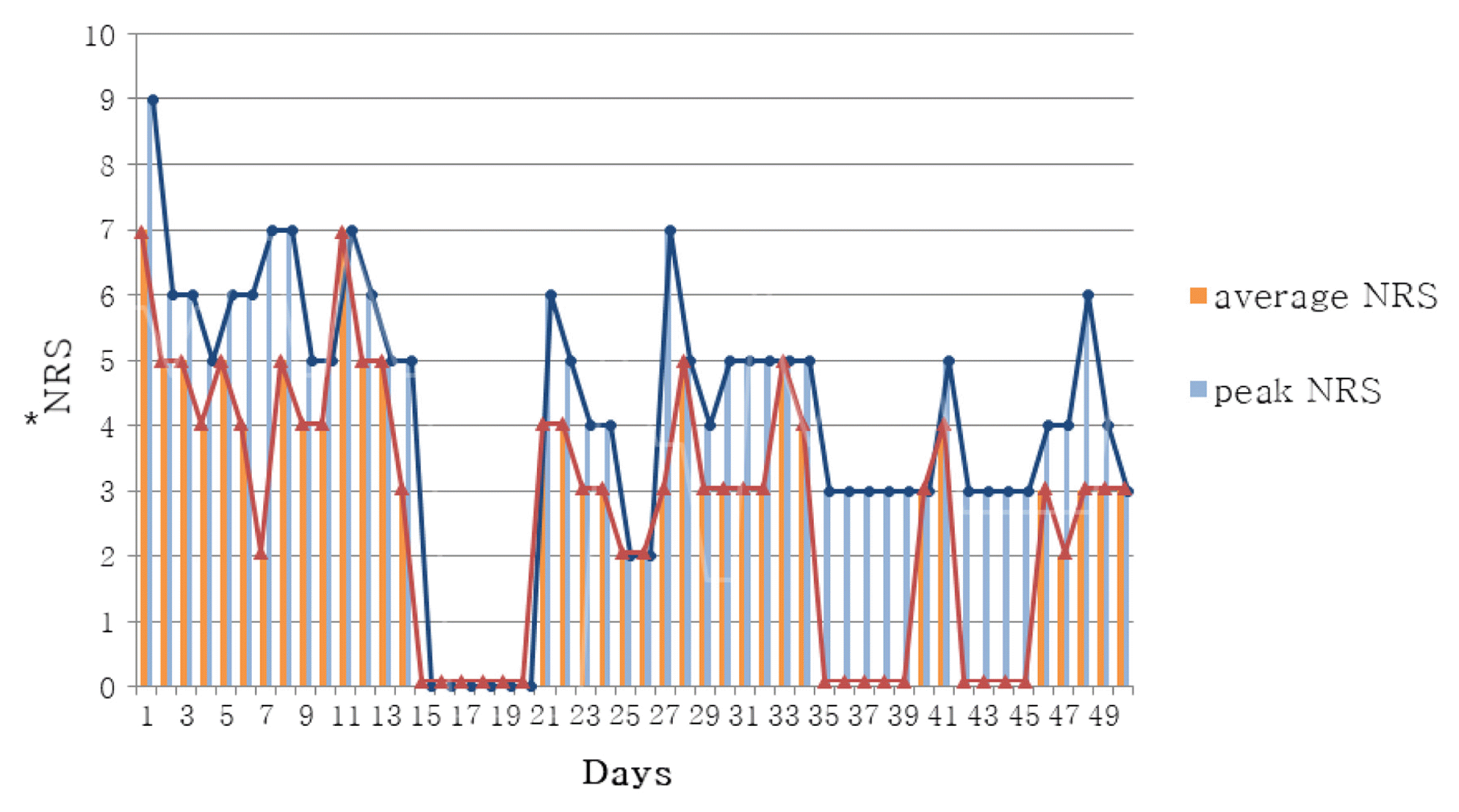
Changes of NRS Scores for Headaches
*The NRS (Numerical rating scale) score was a self-assessment of the patient’s headache severity.
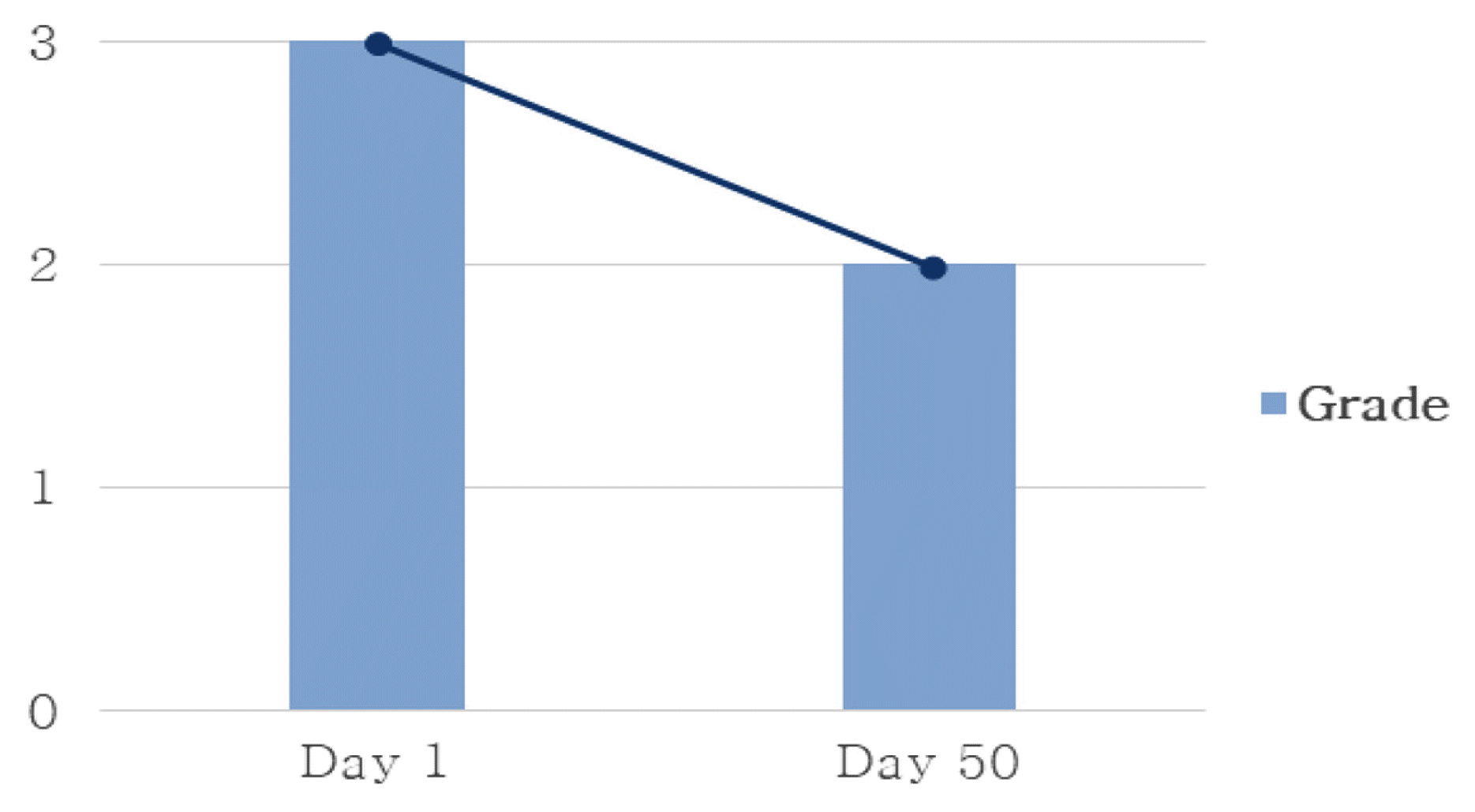
Changes of Sweating
*Grade 0 (No hyperhidrosis): No sweat at all.
Grade 1 (Mild hyperhidrosis): The skin is moderately moist.
Grade 2 (Moderate hyperhidrosis): Pearls of sweat form on the skin.
Grade 3 (Severe hyperhidrosis): Sweat drips.
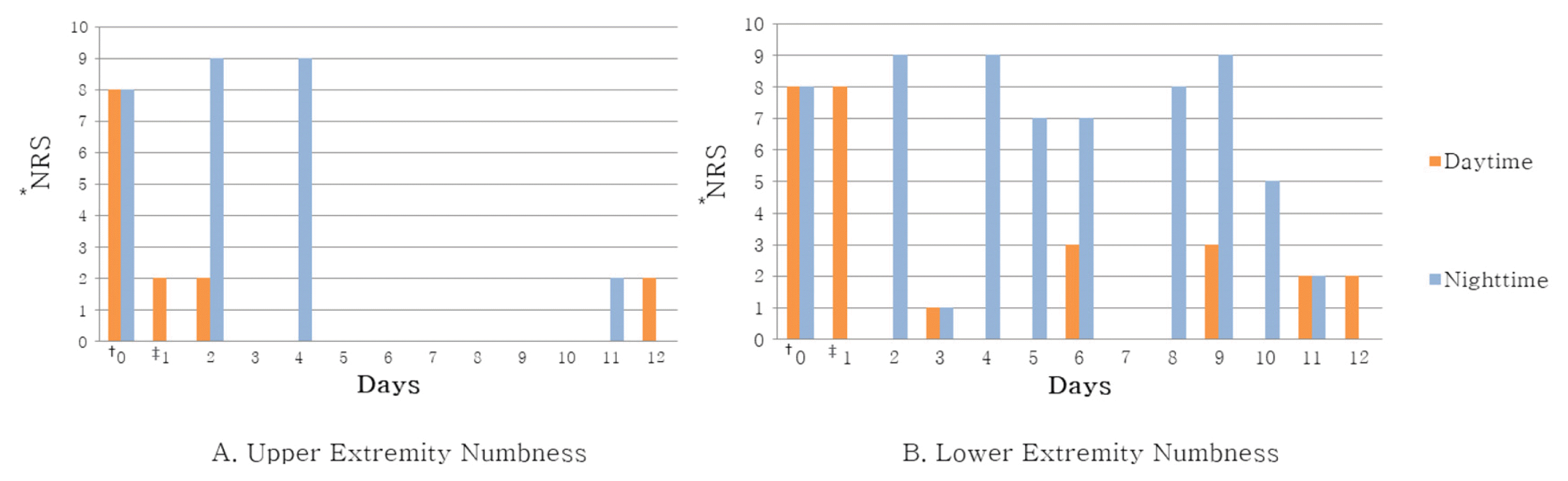
Changes of Numbness in Extremities During Day and Night Time (A) Upper Extremity Numbness (B) Lower Extremity Numbness
* Numerical Rating Scale
†One day before admission
‡Day of admission
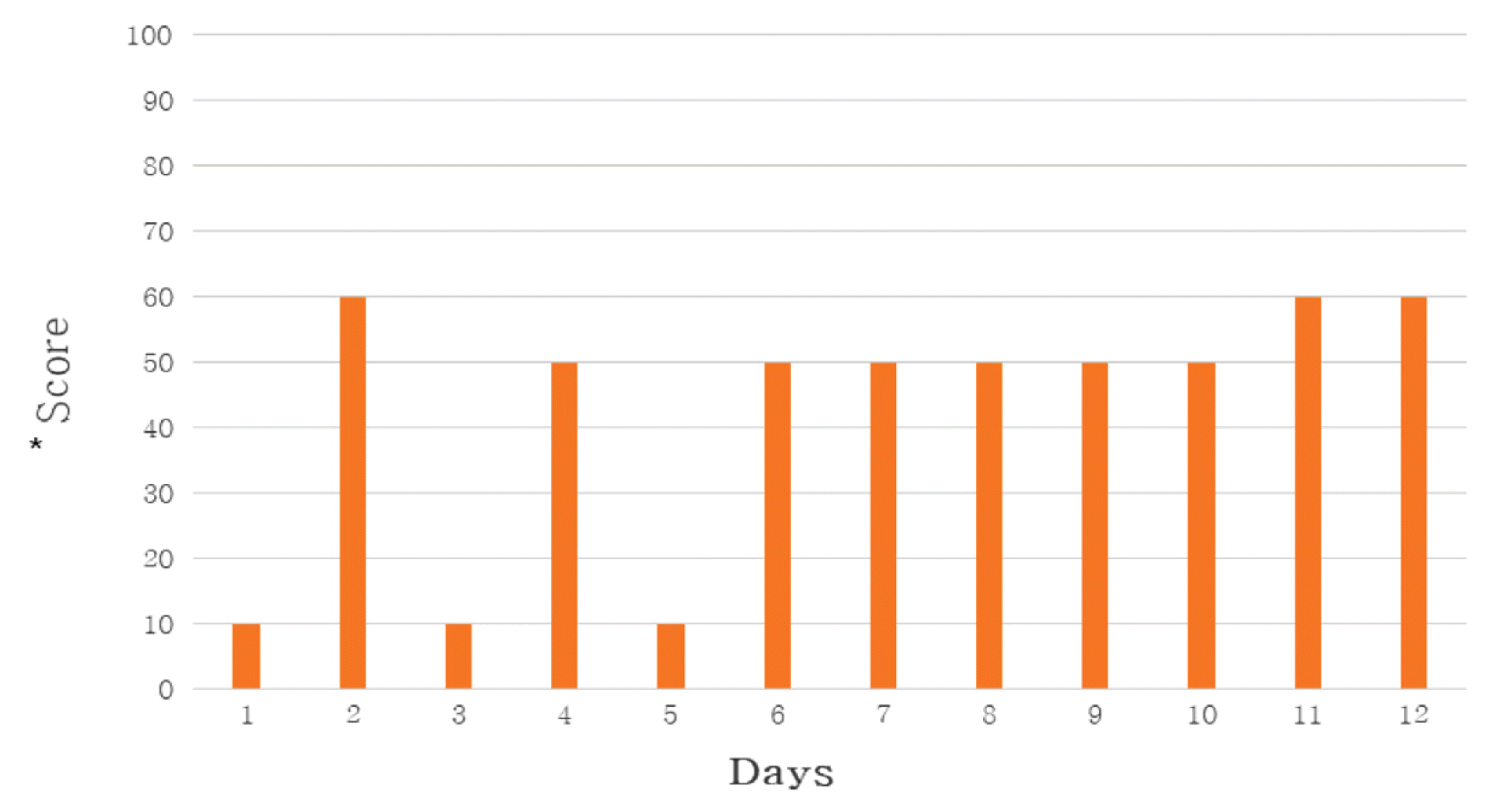
Self-evaluation of General Weakness
*The score is a subjective rating scale on general weakness.
A score of 0 indicates the lowest level of energy, and 100 indicates the highest level of energy.