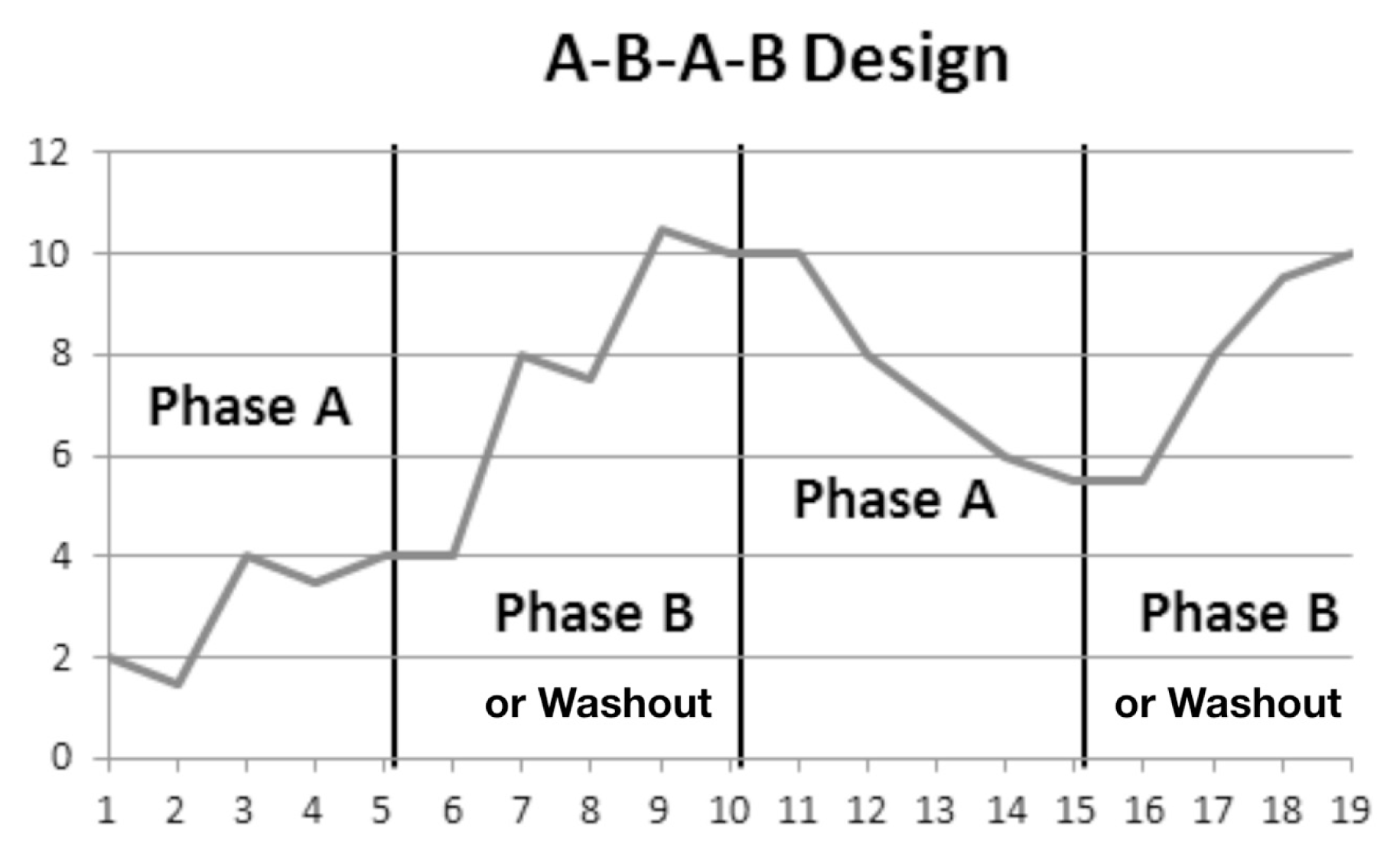
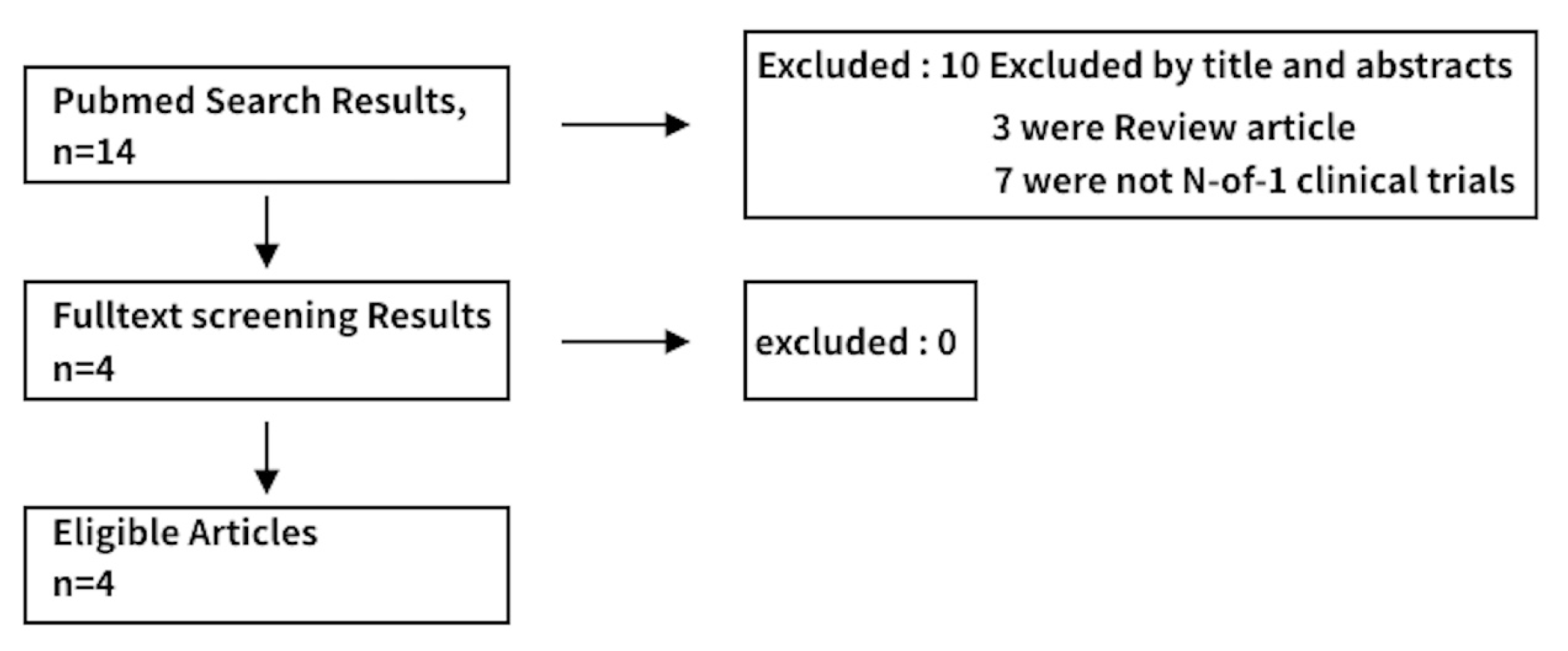
References
1. Akobeng AK. Understanding randomised controlled trials. Archives of Disease in Childhood 2005;90(8):840–844.
2. Spieth PM, Kubasch AS, Penzlin AI, Illigens BM, Barlinn K, Siepmann T. Randomized controlled trials - a matter of design. Neuropsychiatric Disease and Treatment 2016;12:1341–9.
3. Suresh K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. Journal of Human Reproductive Sciences 2011;4(1):8–11.
4. Jones DS, Podolsky SH. The history and fate of the gold standard. Lancet 2015;385(9977):1502–3.
5. Chao J, Dai Y, Verpoorte R, Lam W, Cheng YC, Pao LH, et al. Major achievements of evidence-based traditional Chinese medicine in treating major diseases. Biochemical Pharmacology 2017;139:94–104.
6. Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ 1996;312(7023):71–2.
7. Guyatt G, Sackett D, Taylor DW, Chong J, Roberts R, Pugsley S. Determining optimal therapy--randomized trials in individual patients. The New England Journal of Medicine 1986;314(14):889–92.
8. Guyatt GH, Keller JL, Jaeschke R, Rosenbloom D, Adachi JD, Newhouse MT. The n-of-1 randomized controlled trial: clinical usefulness. Our three-year experience. Annals of Internal Medicine 1990;112(4):293–9.
9. Shaffer JA, Kronish IM, Falzon L, Cheung YK, Davidson KW. N-of-1 Randomized Intervention Trials in Health Psychology: A Systematic Review and Methodology Critique. Annals of Behavioral Medicine 2018;52(9):731–742.
10. Mirza RD, Punja S, Vohra S, Guyatt G. The history and development of N-of-1 trials. Journal of the Royal Society of Medicine 2017;110(8):330–340.
11. Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Personalized Medicine 2011;8(2):161–173.
12. Schork NJ. Personalized medicine: Time for one-person trials. Nature 2015;520(7549):609–11.
13. Terasawa K. Evidence-based Reconstruction of Kampo Medicine: Part-III-How Should Kampo be Evaluated? Evidence-Based Complementary and Alternative Medicine 2004;1(3):219–222.
14. Li J, Tian J, Ma B, Yang K. N-of-1 trials in China. Complementary Therapies in Medicine 2013;21(3):190–4.
15. Yoshinaga R, Goto Y, Inoue H, Yano H, Nabeshima S, Tahara E. A Case of Lower Back with Extremity Pain Successfully Treated with Kanzobushito. Kampo Medicine 2019;70(2):146–150.
16. Tsubo T, Unita A, Furuta T, Suzuki M, Ueno T, Suzuki T, et al. A Patient with Diffuse Panbronchiolitis Treated with a Combination of Keishikyoshakuyakukasokyoto and Acupuncture. Kampo Medicine 2019;70(2):99–105.
17. Jeung CW, Jeon SW, Kim HK. Reduction of Adverse Effects from Jayeumganghwa-tang for Pegylated Liposomal Doxorubicin and Carboplatin in Recurrent Ovarian Cancer. The Journal of Internal Korean Medicine 2019;40(6):1278–1287.
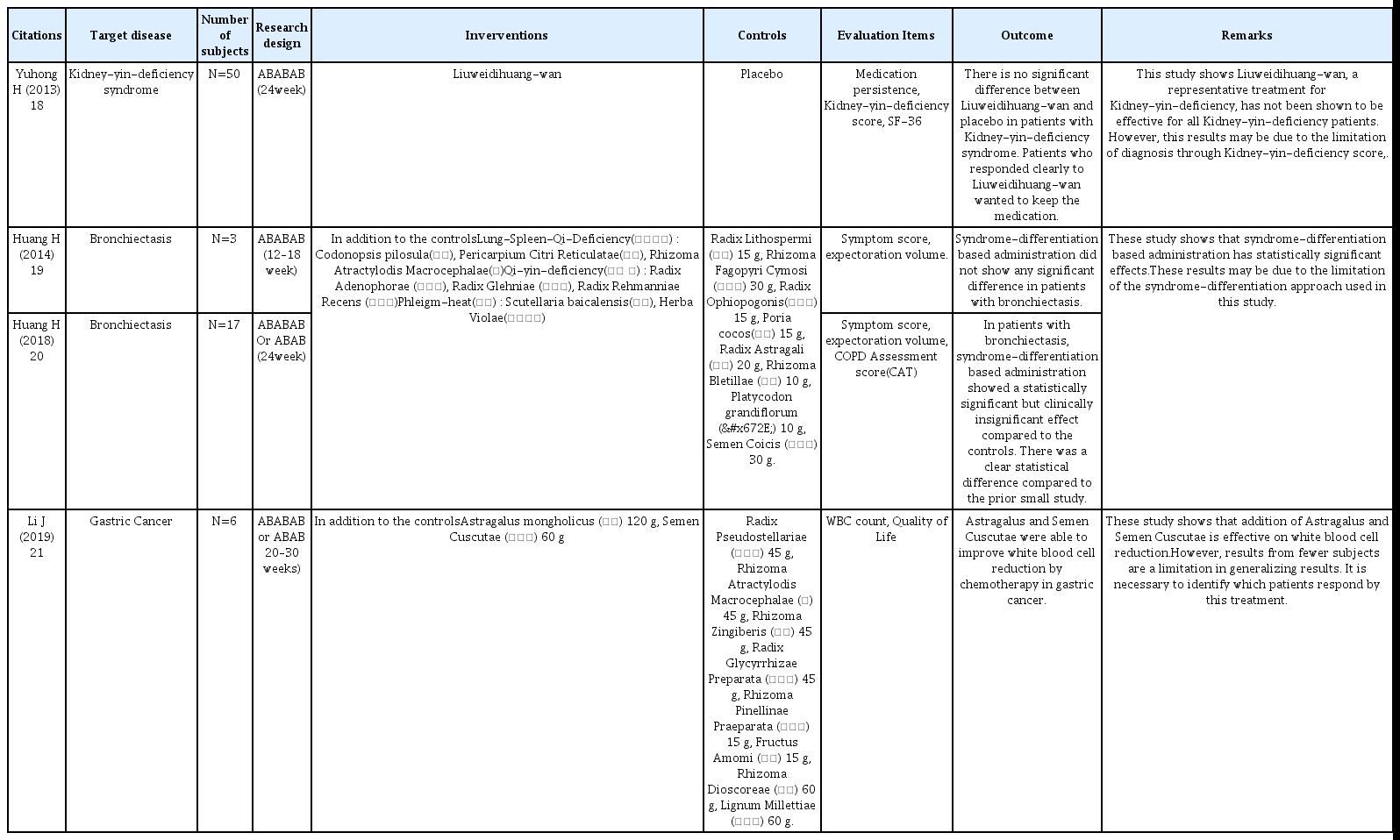
18. Yuhong H, Qian L, Yu L, Yingqiang Z, Yanfen L, Shujing Y, et al. An n-of-1 Trial Service in Clinical Practice: Testing the Effectiveness of Liuwei Dihuang Decoction for Kidney-Yin Deficiency Syndrome. Evidence-Based Complementary and Alternative Medicine 2013;2013827915.
19. Huang H, Yang P, Xue J, Tang J, Ding L, Ma Y, et al. Evaluating the Individualized Treatment of Traditional Chinese Medicine: A Pilot Study of N-of-1 Trials. Evidence-Based Complementary and Alternative Medicine 2014;2014148730.
20. Huang H, Yang P, Wang J, Wu Y, Zi S, Tang J, et al. Investigation into the Individualized Treatment of Traditional Chinese Medicine through a Series of N-of-1 Trials. Evidence-Based Complementary and Alternative Medicine 2018;20185813767.
21. Li J, Niu J, Yang M, Ye P, Zhai J, Yuan W, et al. Using single-patient (n-of-1) trials to determine effectiveness of traditional Chinese medicine on chemotherapy-induced leukopenia in gastric cancer: a feasibility study. Annals of Translational Medicine 2019;7(6):124.
22. Li J, Wei D, Niu J, Yang M, Ge L, Wang X, et al. Potential Facilitators and Barriers to Awareness of N-of-1 Trials for Physicians in Traditional Chinese Medicine. Alternative Therapies In Health And Medicine 2018;24(2):44–49.
23. Xie T, Yu Z. N-of-1 Design and Its Applications to Personalized Treatment Studies. Statistics in Biosciences 2017;9(2):662–675.
24. Li J, Hu JY, Zhai JB, Niu JQ, Kwong JSW, Ge L, et al. CONSORT extension for reporting N-of-1 trials for traditional Chinese medicine (CENT for TCM) : Recommendations, explanation and elaboration. Complementary Therapies in Medicine 2019;46:180–188.
25. Chen J, Huang J, Li JV, Lv Y, He Y, Zheng Q. The Characteristics of TCM Clinical Trials: A Systematic Review of ClinicalTrials.gov. Evidence-Based Complementary and Alternative Medicine 2017;20179461415.
26. Kravitz RL, Schmid CH, Marois M, Wilsey B, Ward D, Hays RD, et al. Effect of Mobile Device-Supported Single-Patient Multi-crossover Trials on Treatment of Chronic Musculoskeletal Pain: A Randomized Clinical Trial. JAMA Internal Medicine 2018;178(10):1368–1377.
27. Mirza RD, Guyatt GH. A Randomized Clinical Trial of n-of-1 Trials-Tribulations of a Trial. JAMA Internal Medicine 2018;178(10):1378–1379.
28. Kravitz RL, Sim I, Duan N. A Case for n-of-1 Trials-Reply. JAMA Internal Medicine 2019;179(3):453.
29. Terasawa K. Evidence-based Reconstruction of Kampo Medicine: Part II-The Concept of Sho. Evidence-Based Complementary and Alternative Medicine 2004;1(2):119–123.
30. Duan N, Kravitz RL, Schmid CH. Single-patient (n-of-1) trials: a pragmatic clinical decision methodology for patient-centered comparative effectiveness research. Journal of Clinical Epidemiology 2013;66(8 Suppl):S21–8.
31. Zucker DR, Ruthazer R, Schmid CH. Individual (N-of-1) trials can be combined to give population comparative treatment effect estimates: methodologic considerations. Journal of Clinical Epidemiology 2010;63(12):1312–1323.
32. Takahashi K, Yoshino T, Maki Y, Ishiuchi K, Namiki T, Ogawa-Ochiai K, et al. Identification of glycyrrhizin metabolites in humans and of a potential biomarker of liquorice-induced pseudoaldosteronism: a multi-centre cross-sectional study. Archives of Toxicology 2019;93(11):3111–3119.
33. Sánchez-Vidaña DI, Rajwani R, Wong MS. The Use of Omic Technologies Applied to Traditional Chinese Medicine Research. Evidence-Based Complementary and Alternative Medicine 2017;20176359730.