Clinical Efficacy and Safety of Yukgunja-tang for Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis
Article information
Abstract
Objectives
The purpose of this meta-analysis was to evaluate the effects of Yukgunja-tang(YGJT, Rikkunshito, Liujunzi) on Gastroesophageal Reflux Disease(GERD).
Methods
Sixteen electronic databases were used to search for studies published through March 2019, and a randomized controlled study was conducted to study the effects of YGJT or modified YGJT on GERD. Study quality was assessed using the risk bias tool provided by Cochran, and data analysis was performed using Review Manager 5.3.0 software
Results
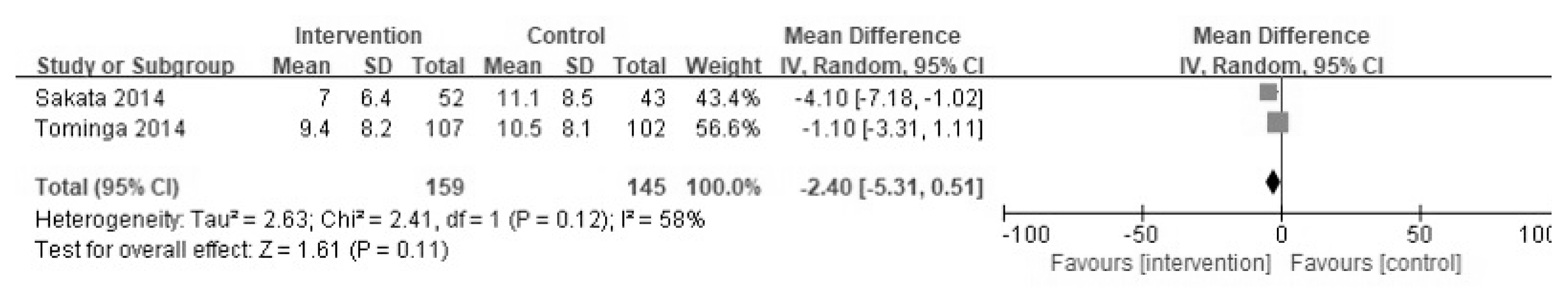
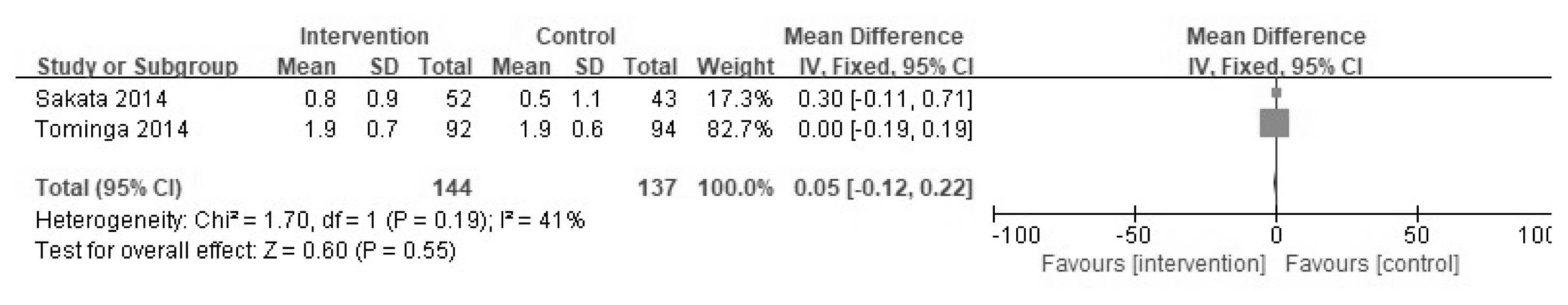
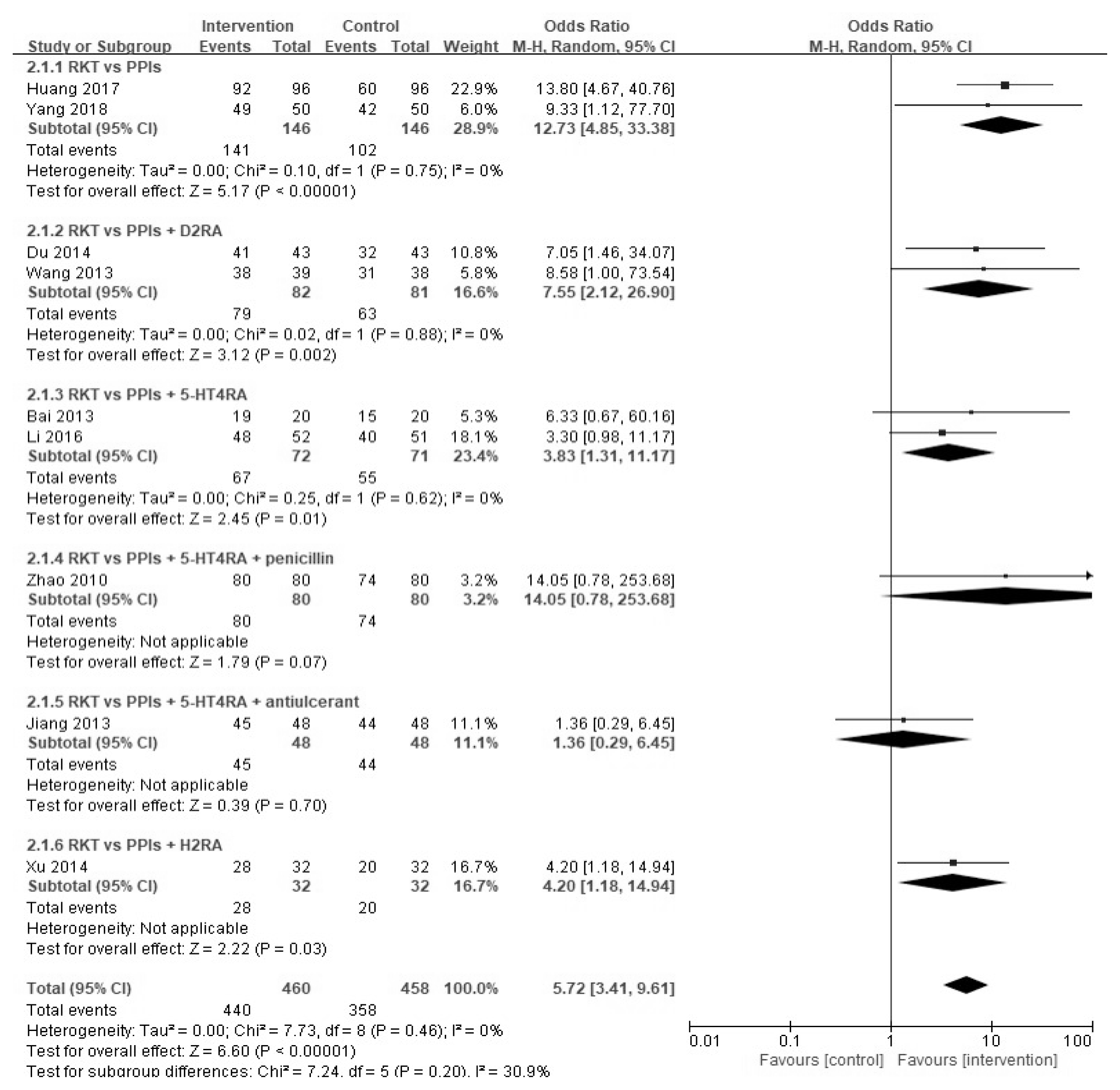
Two-hundred and forty articles were initially searched, and 13 studies that satisfied the study criteria were evaluated qualitatively; 11 of the 13 were included in the meta-analysis. In the two studies, the effects of YGJT and a placebo were compared. Meta-analysis showed that YGJT significantly improved FSSG (Frequency Scale for the Symptoms of GERD) scores, but not GSRS (Gastrointestinal Symptom Rating Scale) scores (FSSG: MD −2.40, 95% CI [−5.31, 0.51], p=0.11, GSRS: MD 0.05, 95% CI [−0.12, 0.22], p=0.55). Meta-analysis of nine studies comparing the efficacies of YGJT and conventional medicine showed that YGJT had a significant clinical effect (OR 5.72; 95% CI [3.41, 9.61]; I2 p<0.00001).
Conclusion
This study suggests that YGJT effectively relieves the symptoms of GERD. Unfortunately, owing to the small sample sizes, limitations of several methodological qualities, we believe large-scale clinical studies with less bias will provide evidence of qualitative improvement.
Introduction
Gastroesophageal Reflux Disease (GERD) is commonly encountered in clinical practice1). The prevalence of GERD in Korea increased from 3.4% in 1998 to 5–29.2% in 20052). The Montreal definition of GERD is a condition that develops when reflux of stomach contents causes troublesome symptoms and/or complications3). Epidemiologic estimates of the prevalence of GERD are based primarily on the typical symptoms of heartburn and acid regurgitation1). Empiric PPI therapy provides a reasonable means of confirming the presence of GERD when it is suspected in patients with typical symptoms1). In Japan, the traditional medication Yukgunja-tang (YGJT, Rikkunshito, Liujunzi) has been approved for medicinal use, and is widely used to treat patients with upper gastrointestinal (GI) symptoms4).
Although YGJT is used for clinical treatment of GERD in China and Japan and related clinical studies are underway, only random studies on the effects of YGJT on functional dyspepsia5) and in animal models6) have been conducted in Korea. Therefore, the aim of this meta-analysis was to systematically review the efficacy and safety of YGJT when used to treat GERD.
Methods
1. Data Sources and Search Strategy
The following electronic databases were searched to identify relevant studies uploaded through March 2019: EMBASE, MEDLINE, the Cochrane Library (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL), PubMed, six Korean medical databases (Korea Med, the Korean Medical database, the Korean Studies Information Service System, the Korea Institute of Science and Technology Information, NDSL, and the Oriental Medicine Advanced Searching Integrated System), three Chinese medical databases (the Chinese Medical Database [CNKI], Chongqing VIP Chinese Science and Technology Periodical Database [VIP], and the WanFang Database), and two Japanese databases (J Global and J-Stage). The search terms used were as follows: (Liujunzi OR Rikkunshito OR Yukgunja tang OR Yukkunja tang) AND (gastroesophageal reflux disease OR reflux esophagitis OR nonerosive gastroesophageal reflux disease OR Barrett’s esophagus OR Heartburn OR PPI-refractory OR Refractory OR Regurgitation OR Globus pharyngitis) in Korean, English, Chinese, and Japanese. The databases used in this study were independently searched manually.
2. Selection
1) Types of Studies
All randomized controlled trials (RCTs) were included. Observational, cohort, case-control, case series, qualitative, laboratory studies, and uncontrolled trials were excluded.
2) Types of Participants
GERD was diagnosed using published diagnostic criteria. No restrictions were placed on race, age, or sex.
3) Types of Interventions
Studies that used YGJT or a modified YGJT were included. Modified YGJT prescribed according to traditional Korean medical syndrome definitions was deemed acceptable for the purposes of this study and was defined by practitioners as the addition of herbs to the herbs used to prepare YGJT that resulted in the same effects as YGJT.
4) Types of Comparisons
We included all types of control intervention, including placebo and conventional medication.
3. Outcome Measures
Outcomes were assessed using efficacies, Frequency Scale for the Symptoms of Gastroesophageal reflux disease (FSSG), or Gastrointestinal Symptom Rating Scale (GSRS) during or after intervention, recurrence rate, and adverse effects as a dichotomous and continuous outcome.
4. Data Extraction and Risk-of-Bias Assessment
Two authors (SWP and SWL) performed the data extraction and collated information independently on participants, interventions, treatment durations, outcomes, and results. Any disagreement between the two authors was resolved by discussion. Risk of bias was assessed using the following seven criteria from Cochrane Collaboration’s risk of bias tool.
5. Data Synthesis
The statistical analysis was conducted using Review Manager 5.3.0 software from the Cochrane Collaboration for Windows (The Nordic Cochrane Center, Copenhagen, Denmark). Differences between intervention and control groups were assessed. Dichotomous outcomes were analyzed using the odds ratio (OR) with 95% confidence intervals (CIs) for relative effect. For continuous outcomes, we used the mean difference (MD) with 95% confidence intervals (CIs) to measure effect. Heterogeneity was assessed using the chi-squared test and the I2 test. A fixed effects model was used when studies were homogeneous, and a random effects model was used when there was heterogeneity (I2 > 50%) between studies.
Results
1. Description of the Included Trials
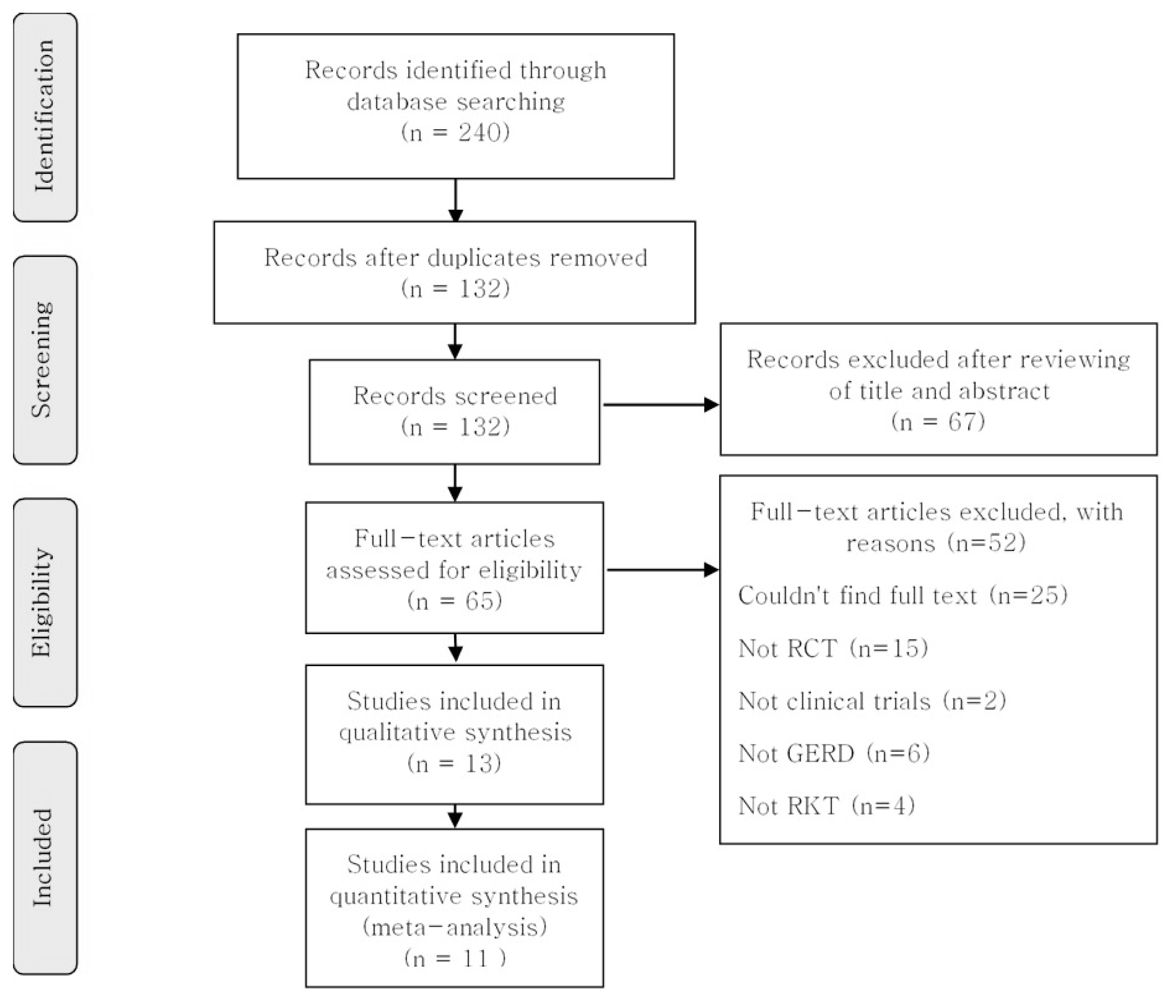
A total of 240 studies were originally identified by searching 16 databases using search terms and search strategies. Of these, 108 were excluded for duplication. The remaining 132 studies were reviewed with a focus on titles and abstracts, and 67 were primarily excluded. Subsequently, another 52 studies were excluded for the following reasons: 25 articles could not find the full text, 15 were not randomized, 2 did not include a clinical trial, 6 did not discuss GERD, and 4 did not use YGJT. Finally, 13 studies were selected, and 11 were included in the meta-analysis. The flow chart of the selection process is provided in Figure 1.
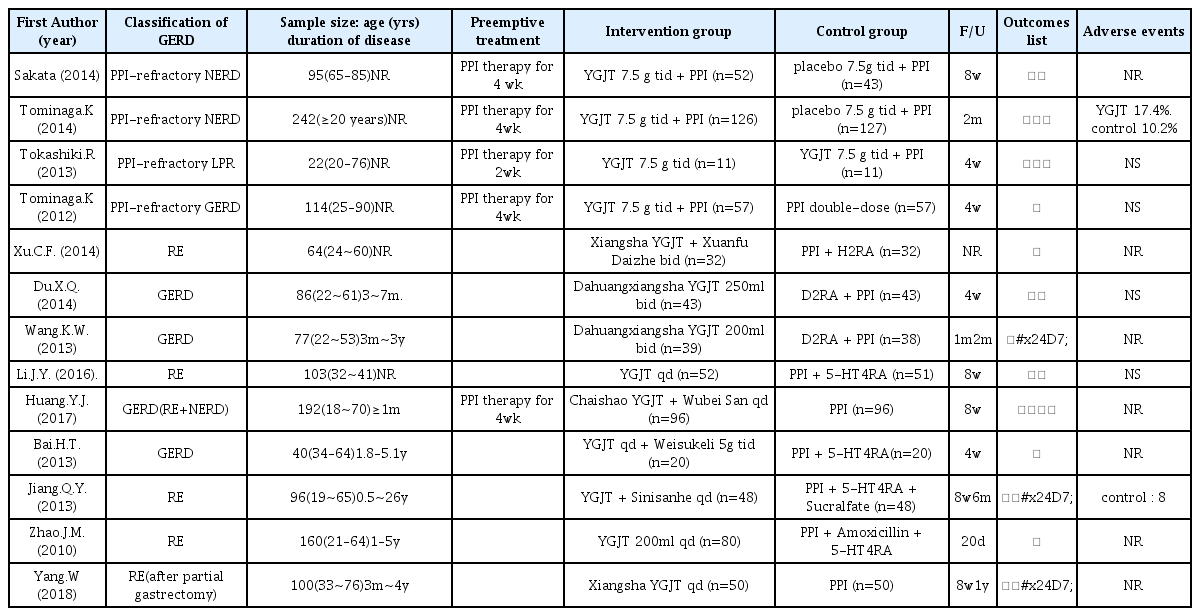
Characteristic data from the 13 studies are summarized in Table 1. Four of the 13 were published in Japan and 9 were published in China between 2010 and 2018. Four studies were reported as GERD, five as RE, one as PPI-refractory GERD, two as PPI-refractory NERD, and one as PPI-refractory LPR (laryngopharyngeal reflux). Sample sizes ranged from 64 to 242, and patients’ ages from 18 to 90 years. Seven studies reported disease durations from 1 month to 26 years, and disease durations were not mentioned in six studies.
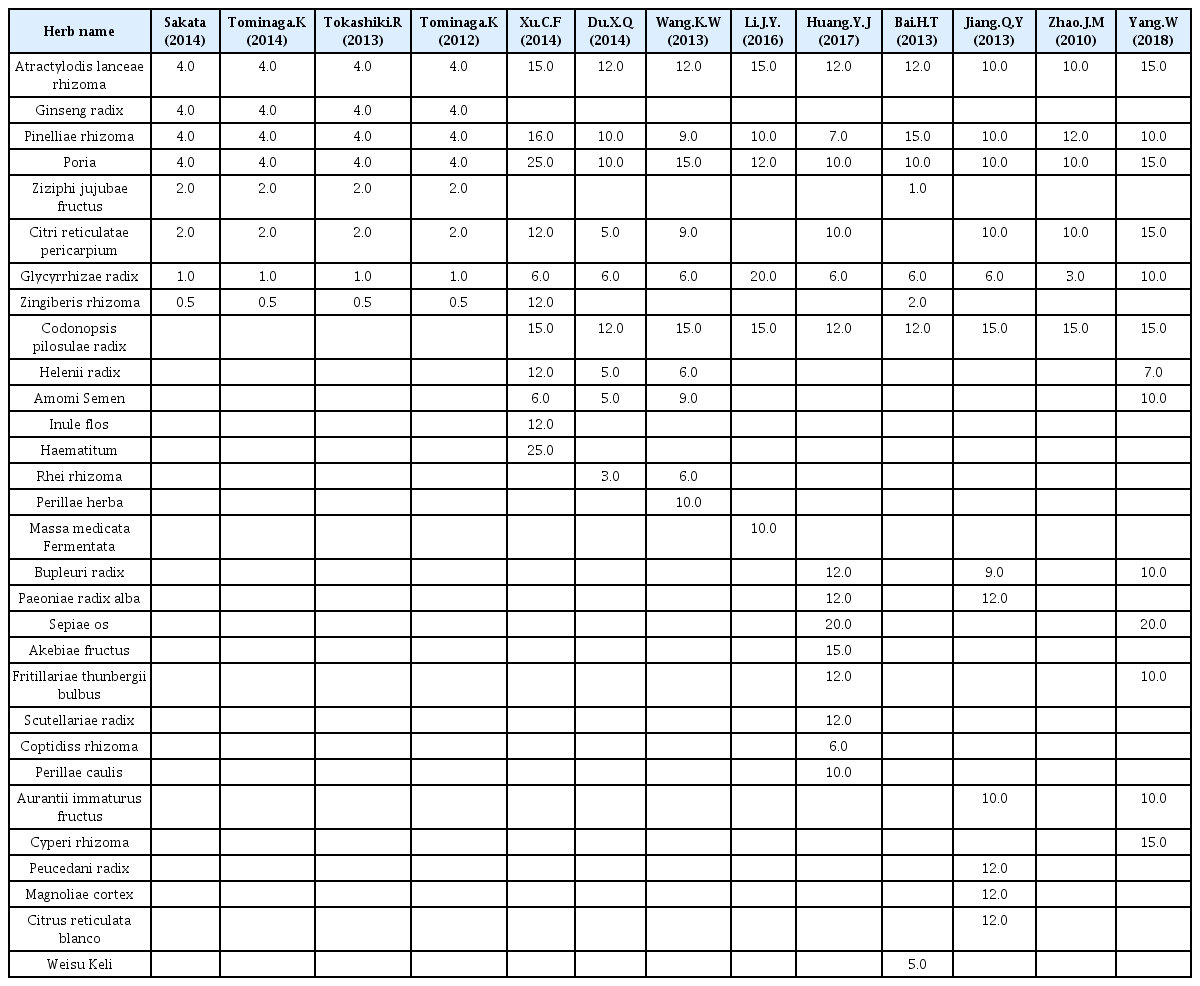
Two studies compared YGJT with a placebo. One study compared YGJT plus PPI with double-dose PPI, and one compared the effects of YGJT and YGJT plus PPI. The remaining 9 studies compared the effects of YGJT with those of conventional medicines. Therapy durations ranged from 20 days to 8 weeks. Thirteen studies provided details of the herbal formulae of YGJT, and the constituents of the herbal formulae of YGJT are listed in Table 2.
2. Risk of Bias
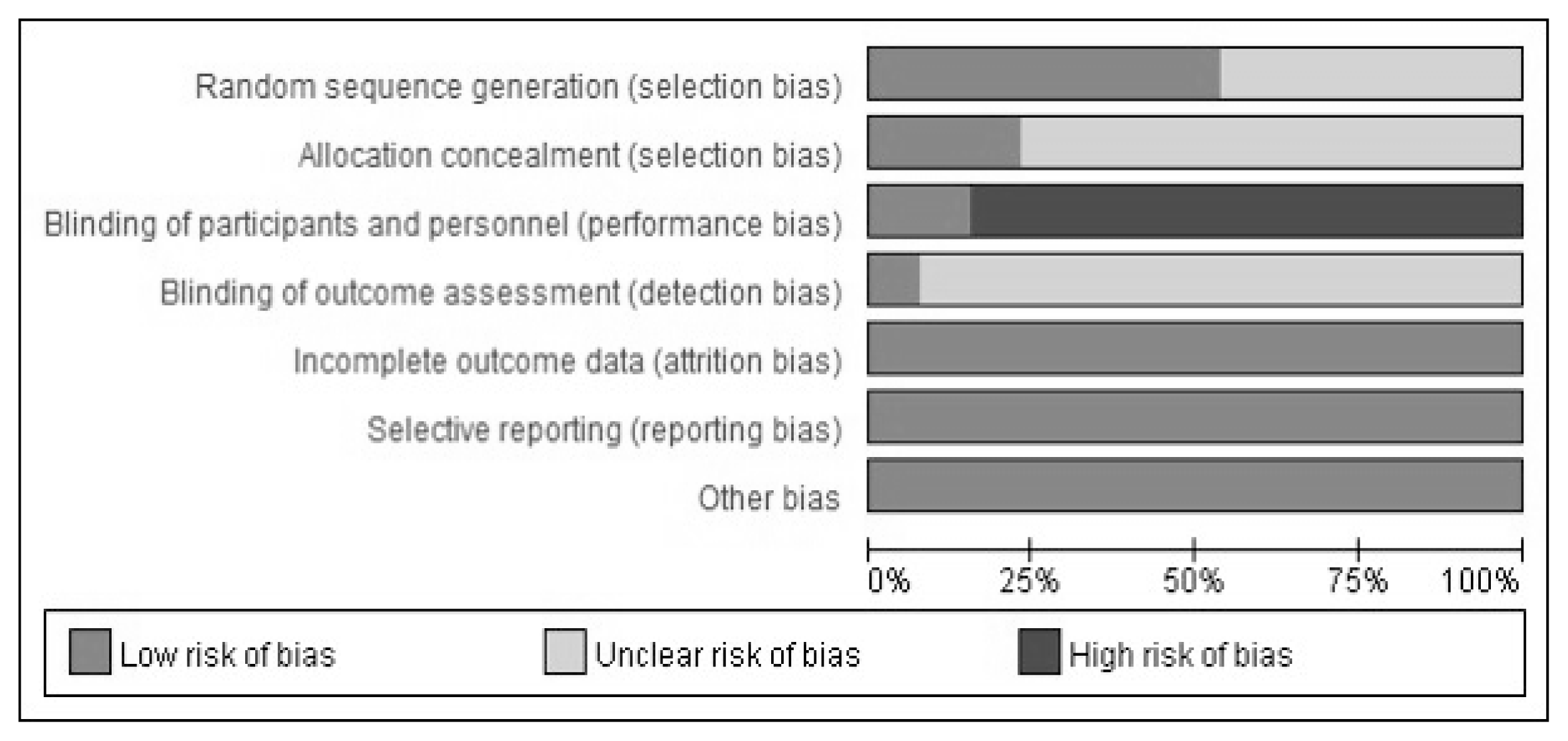
All studies were described as ‘randomized’, but only seven studies reported the method of random sequence generation. Three studies described allocation concealments. Eleven studies had high risks of bias for participant and personnel blinding, and two studies had low risk. Only one trial used outcome assessment blinding. All included studies reported complete outcome data. All studies had a low risk of selective reporting bias; four studies were published protocols and nine studies included all expected outcomes (Figure 2, 3).
3. Outcome Measurements
YGJT plus PPI versus double-dose PPI and YGJT versus YGJT plus PPI were excluded from the meta-analysis because these were conducted in one study each.
1) YGJT versus Placebo
(1) Symptom Rating Scores
Two of the 13 studies compared the effects of YGJT versus a placebo. FSSG and GSRS scales were used to evaluate YGJT efficacy. Of the 348 patients, 178 received YGJT plus PPI and 170 received a placebo plus PPI. The FSSG scores of these two groups resulted in moderate heterogeneity (I2 = 58%), so a random effects model was adopted to produce the results (FSSG: MD −2.40, 95% CI [−5.31, 0.51], p =0.11) (Figure 4). The fixed effects model was adopted for GSRS scores because I2 = 41%, (GSRS: MD 0.05, 95% CI [−0.12, 0.22], p =0.55) (Figure 5). The FSSG score had statistically significant results in the YGJT group, but the GSRS score did not show a statistically significant difference in the two studies.
2) YGJT versus Conventional Medicine
Nine11–19) studies on YGJT and conventional medicine included improvements in symptom scores and suggested effects of the treatment for each symptom; these comprised 918 patients (460 in the YGJT groups and 458 in the conventional medicine groups). In two studies, scores for each symptom were presented. Symptoms included heartburn, acid regurgitation, sternalgia, and reflux. Four studies reported efficacy under gastroscopy, but one study was excluded because its scoring criteria differed from those used in the three other studies12,15,17). In one study, reflux symptom scores were excluded from the meta-analysis.
PPIs (Proton pump inhibitors), PPIs+D2RA (D2 receptor antagonists), PPIs+5-HT4RA (5-HT4 receptor agonists), PPIs+5-HT4RA + penicillin, PPIs + 5-HT4RA + antiulcerant, and PPIs+H2RA (H2 receptor antagonists) were used in the studies; PPIs contained omeprazole, rabeprazole, lansoprazole, and esomeprazole; D2RA contained domperidone; 5-HT4RA contained mosapride and cisapride; H2RA contained famotidine; penicillin contained amoxicillin; and antiulcerant contained sucralfate.
(1) Clinical Efficacy
In a comparative study of YGJT group versus conventional medicine group, subgroup analysis was conducted for statistical heterogeneity and response rate for these two groups, both separately and combined. Nine studies compared the effects of YGJT and conventional medicine, and a favorable effect was found for the response rate to YGJT by meta-analysis (OR 5.72; 95% CI [3.41, 9.61]; p < 0.00001). In two RCTs, YGJT had better effects on response rates than PPIs (OR 12.73; 95% CI [4.85, 33.38]; p < 0.00001), and in two other RCTs, YGJT had better response rates than PPIs plus D2RA (OR 7.55; 95% CI [2.12, 26.90]; p = 0.002). In two RCTs that compared YGJT and PPIs plus 5-HT4RA, YGJT had better response rates than 5-HT4RA (OR 3.83; 95% CI [1.31, 11.17]; p =0.01). YGJT with PPIs plus 5-HT4RA plus penicillin, PPIs plus 5-HT4RA plus antiulcerant, and PPIs plus H2RA were found in only one study each, so we could not analyze their effects. (Figure 6).
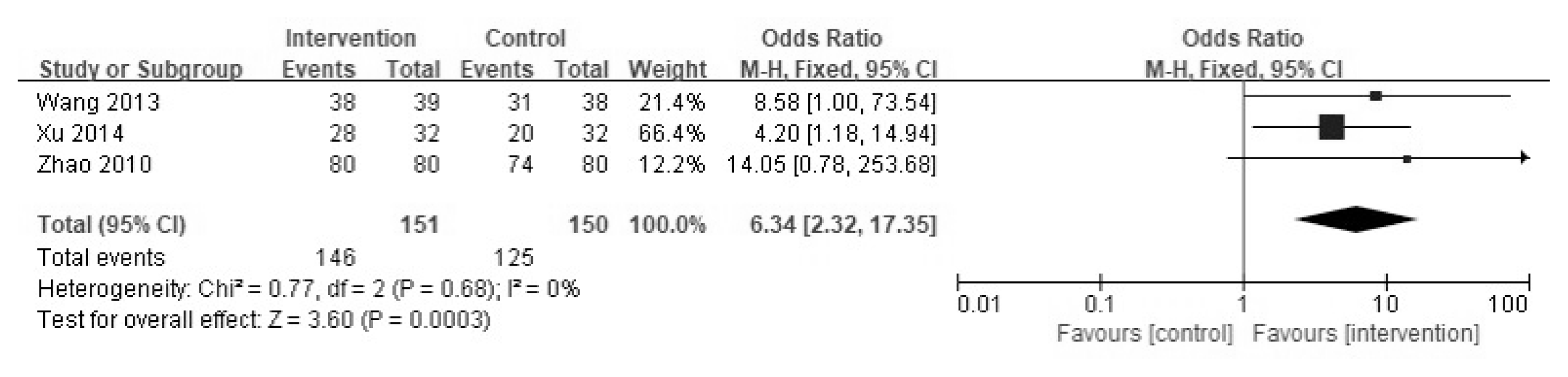
(2) Efficacy under Gastroscopy
Three of the nine studies assessed the efficacies of YGJT and conventional medicine under gastroscopy in a total of 301 patients. The YGJT group contained 151 patients, and the conventional medicine group had 150 patients. Under gastroscopy, YGJT showed significantly better effects than conventional medicine (OR 6.34, 95% CI [2.32, 17.35], p = 0.0003) (Figure 7).
3) Recurrence Rate
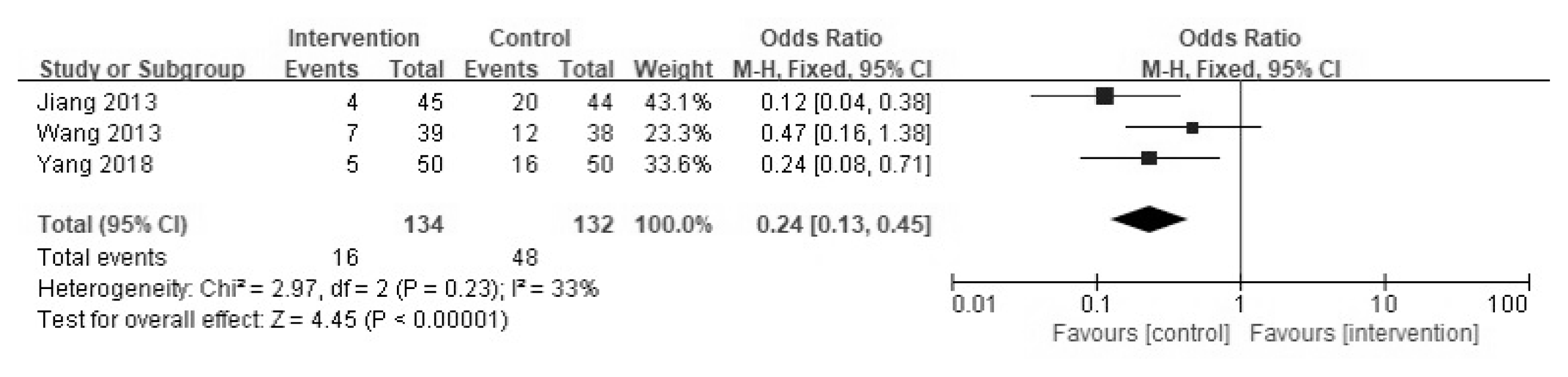
Three of the 13 studies mentioned recurrence rate; recurrences occurred between one and ten months after treatment completion. Although the forest plot showed possible statistical heterogeneity (I2 = 33%), the recurrence rate was significantly decreased in the YGJT group compared to that of the conventional medicine group (OR 0.24, 95% CI [0.13, 0.45] and p < 0.00001) (Figure 8).
4) Adverse Effects
Six studies reported adverse effects during the treatment. Of these, four studies reported no significant adverse effects. In one study, nausea, mild cough, dizziness, and diarrhea were reported in both the YGJT group and the conventional medicine group. One study mentioned the number of patients that experienced adverse effects, and eight patients administered conventional medicine reported headache or constipation. In all cases, symptoms disappeared after treatment cessation and did not affect outcomes.
Discussion
GERD is defined as a condition that develops when reflux of stomach contents causes troublesome symptoms and/or complications. Clinically troublesome heartburn is seen in about 6% of the population, and regurgitation was reported in 16% of GERD patients. Heartburn, chest pain, and acid regurgitation are esophageal symptoms of GERD, and may be the presenting symptom on occasion1). GERD is diagnosed using the combinations of symptoms at presentation, endoscopy, ambulatory reflux monitoring, and response to anti-secretory therapy. PPIs are currently the mainstay treatment for GERD. According to treatment guidelines published by the American Journal of Gastroenterology in 2013, an 8-week course of PPIs is the therapy of choice for symptom relief and healing of erosive esophagitis.
Recent studies have shown that YGJT promotes gastric emptying (by promoting the secretion of ghrelin), ameliorates upper gastrointestinal symptoms, and acts as a prokinetic agent in abnormalities of NO-mediated gastric functions such as delayed gastric emptying. In addition, YGJT enhances gastroesophageal clearance. Studies suggest that gastric emptying and esophageal excretion may be enhanced by YGJT. These studies indicate that YGJT can relieve symptoms of GERD.
We selected 13 RCT studies from 16 published electronic databases through March 2019. Two studies compared YGJT plus PPI with a placebo plus PPI. One study compared YGJT with YGJT plus PPI, one study compared YGJT plus PPI with double-dose PPI, and nine studies compared YGJT with conventional medicine. Four of the 13 studies were conducted in Japan, and nine in China. YGJT versus YGJT plus PPI and YGJT plus PPI versus double-dose PPI were excluded from the meta-analysis, because these comparisons were conducted in only one study each. As a result, the meta-analysis was conducted on studies that compared YGJT plus PPI with placebo plus PPI and YGJT versus conventional medicine.
Two studies compared YGJT plus PPI and a placebo plus PPI. GSRS and FSSG scores were used in the Sakata study. In the Tominaga study GSRS, FSSG, and SF-8 scores were used. Patients with PPI-refractory NERD were enrolled in two studies, and did not respond to standard doses of PPIs for more than 4 weeks. GSRS scores were not significant in either study in the meta-analysis. FSSG scores were reported to effectively decrease in the YGJT group by Sakata, but not by Tominaga. The meta-analysis of both studies showed that the YGJT group showed significantly improved FSSG scores.
The nine studies that compared YGJT with conventional medicine were included in the meta-analysis. The conventional medicines used were PPI, D2RA, H2RA, 5-HT4RA, and 5-HT4RA plus amoxicillin or sucralfate. Clinical efficacies were evaluated by scoring heartburn, throat symptoms, acid regurgitation, sternalgia, esophageal edema, and ulcer healing as determined by gastroscopy. In addition, YGJT had a statistically significant effect on heartburn, acid regurgitation, and sternalgia symptom scores. Clinical effects, gastroscopy determined efficacy, and recurrence rate were more effective in the YGJT group than in the conventional medicine group. In addition, recurrence rate was also significantly decreased in the YGJT group. YGJT was not found to be associated with any serious side effects. All reported side effects also disappeared after discontinuing medicines.
Although all RCT studies performed randomization, most did not provide details of the random sequence generation methods used. Furthermore, most studies did not perform allocation concealment or blinding of patients, practitioners, or accessors. These shortcomings might have resulted in selection, performance, or detection bias, and might have exaggerated therapeutic effects. These studies should be revised, because bias could reduce the reliability of the research.
Several limitations of this systematic review should be considered. First, the RCTs included small sample sizes and low, questionable methodological qualities. Second, while domestic and foreign studies were originally extracted, no Korean or western countries studies were included, which means that generalizations of our results are limited by racial bias.
In conclusion, this meta-analysis shows that YGJT improves symptoms in GERD patients. In this study of systematic review(SR), there were few comparative studies of the placebo group and the YGJT group. Therefore, further study with a placebo group is needed. Recent studies on GERD have reported research on Banxiaxiexin-tang, but research on acupuncture has been difficult to find. Clinical studies on acupuncture alone, or a combination of acupuncture and YGJT, should be performed. Although this study has focused on the analysis of the effects of YGJT on GERD, an acupuncture and YGJT combination treatment in GERD patients who have not responded to pharmacological treatments can be expected to be more effective.
Conclusions
The results of this systematic review and meta-analysis indicate that YGJT administration is effective at relieving symptoms in GERD patients. Unfortunately, owing to the small sample sizes, limitations of several methodological qualities, we believe large-scale clinical studies with less bias will provide evidence of qualitative improvement
Notes
This thesis is a master’s thesis in the Department of Oriental Medicine, Graduate School, Dongguk University, 2019.