A study on the current status and development of the new health technology assessment of Korean medicine field
Article information
Abstract
Objectives
The purpose of this study is to examine the current status of Korean medicine health technology assessment and explore realistic plans to activate it.
Methods
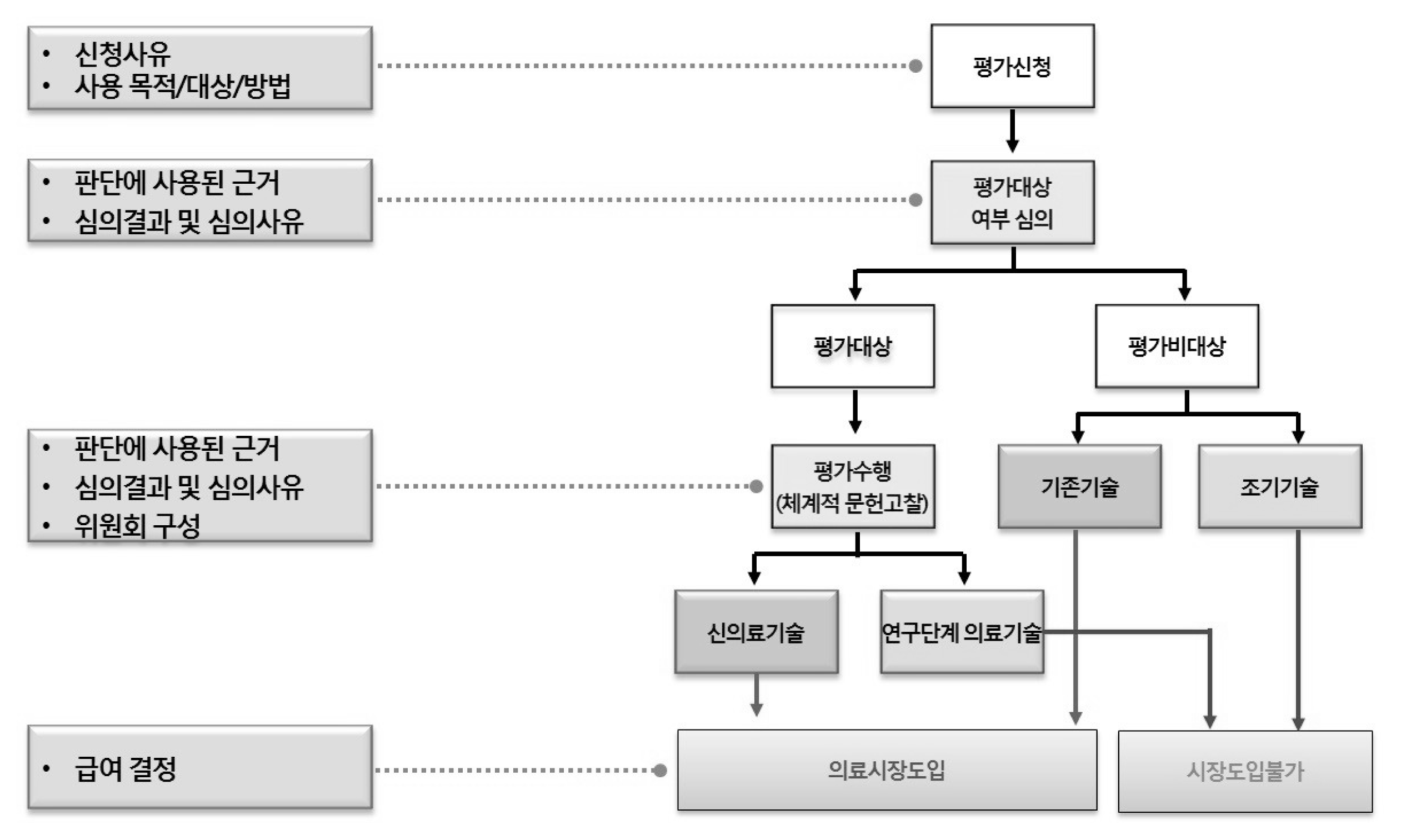
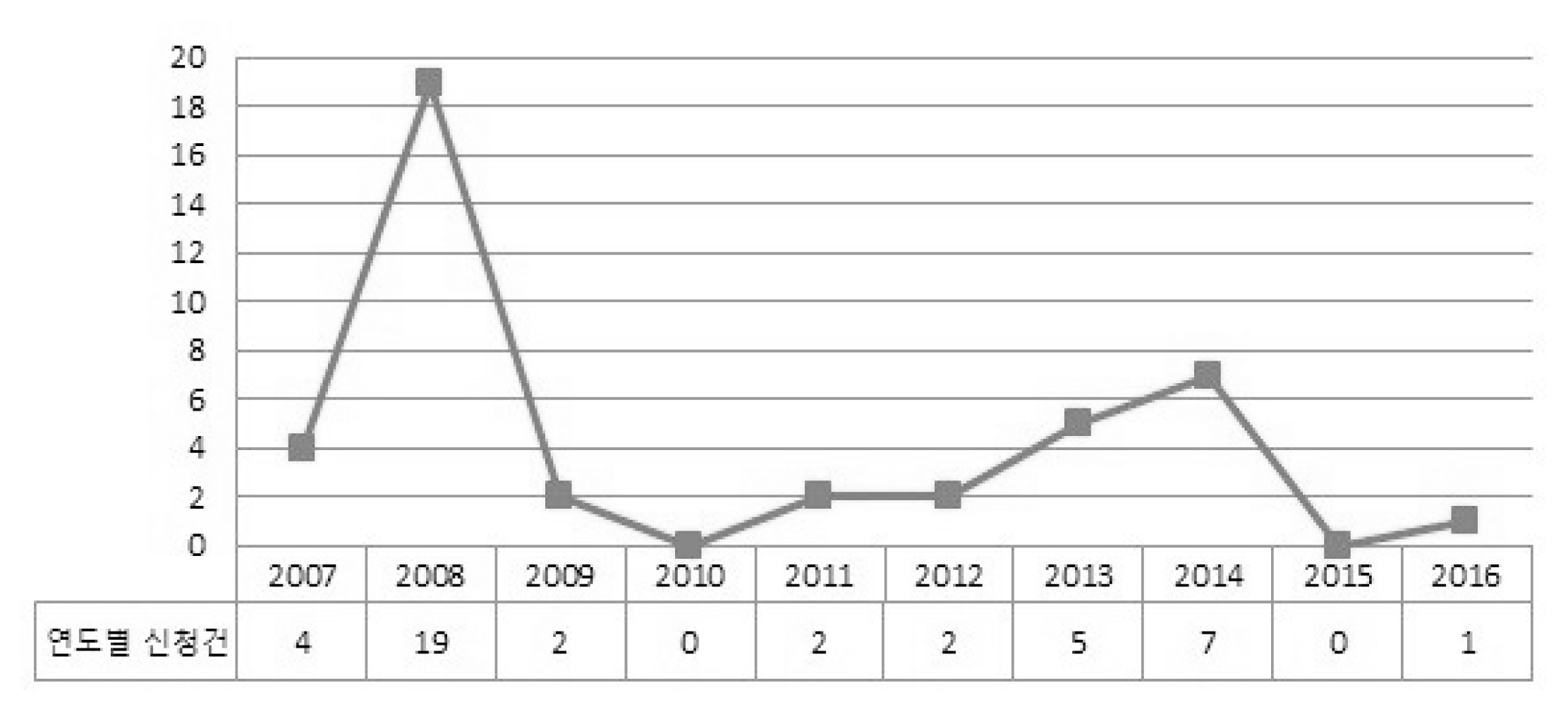
We investigated all the applications for new health technology assessment related to Korean medicine from 2007 to 2016. The several expert meetings were held to draw out the barriers and improvement strategies of the new health technology assessment of Korean medicine field.
Results
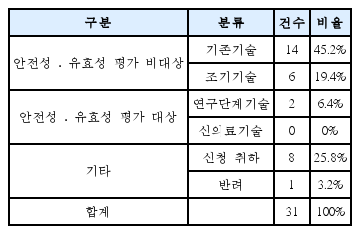
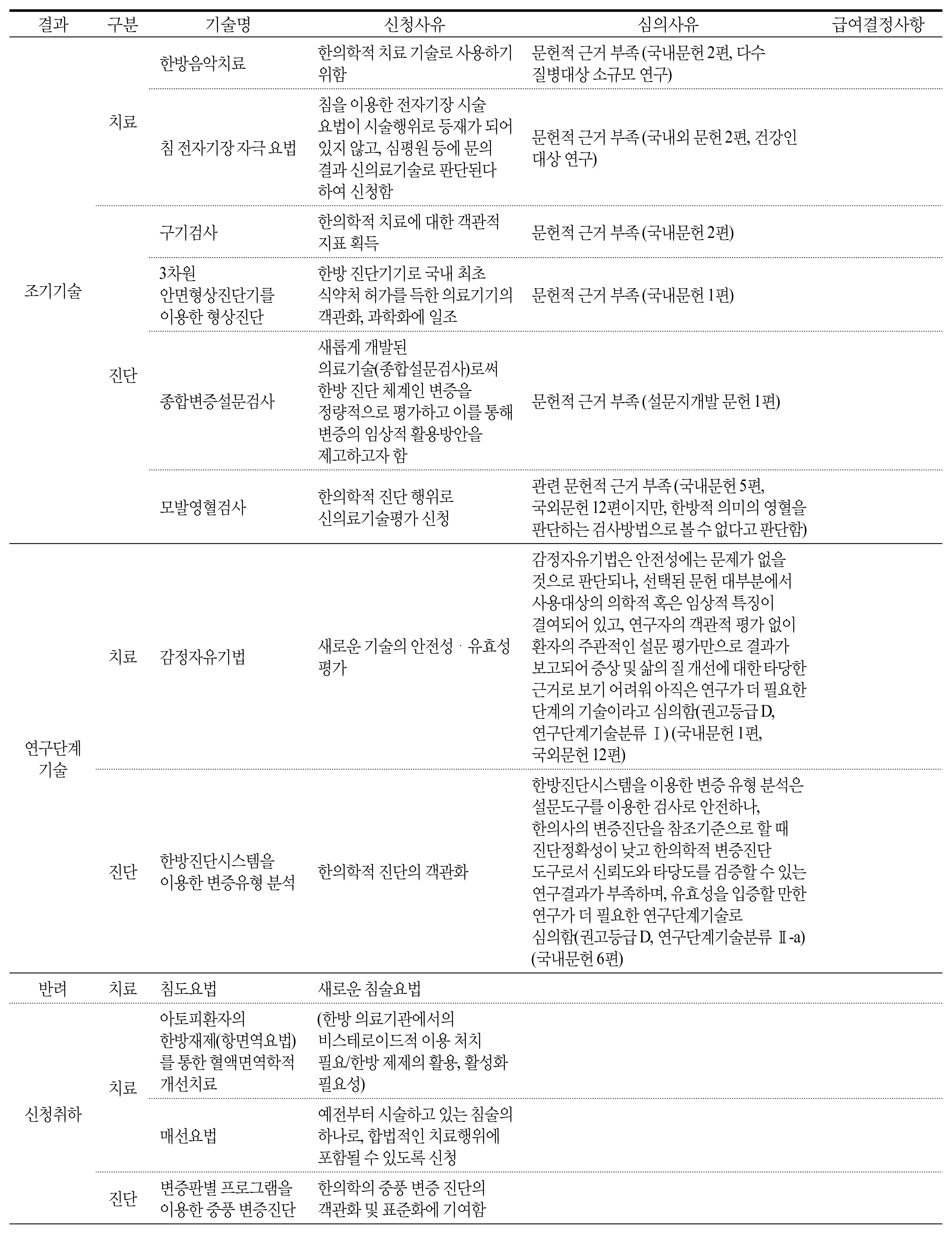
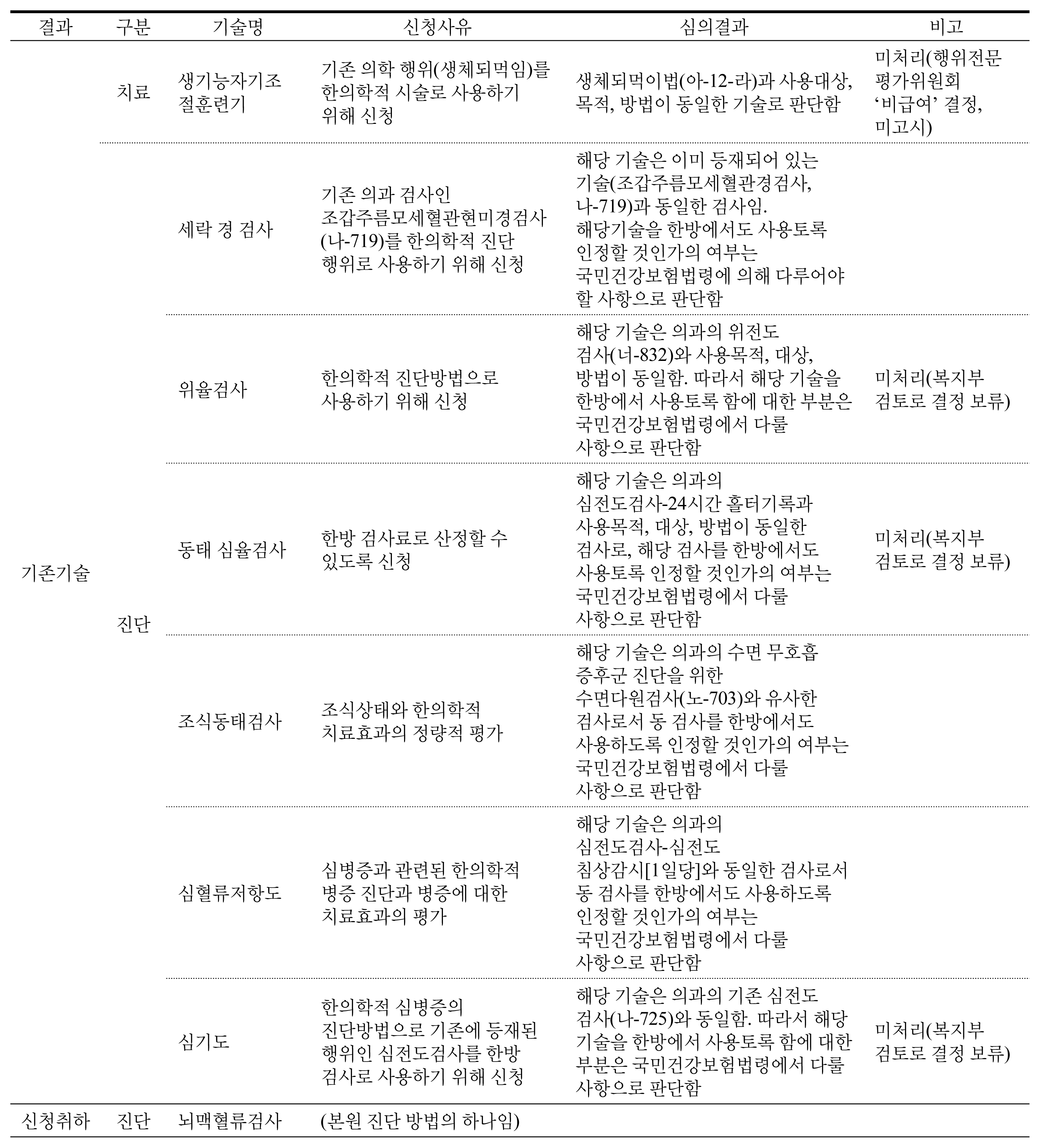
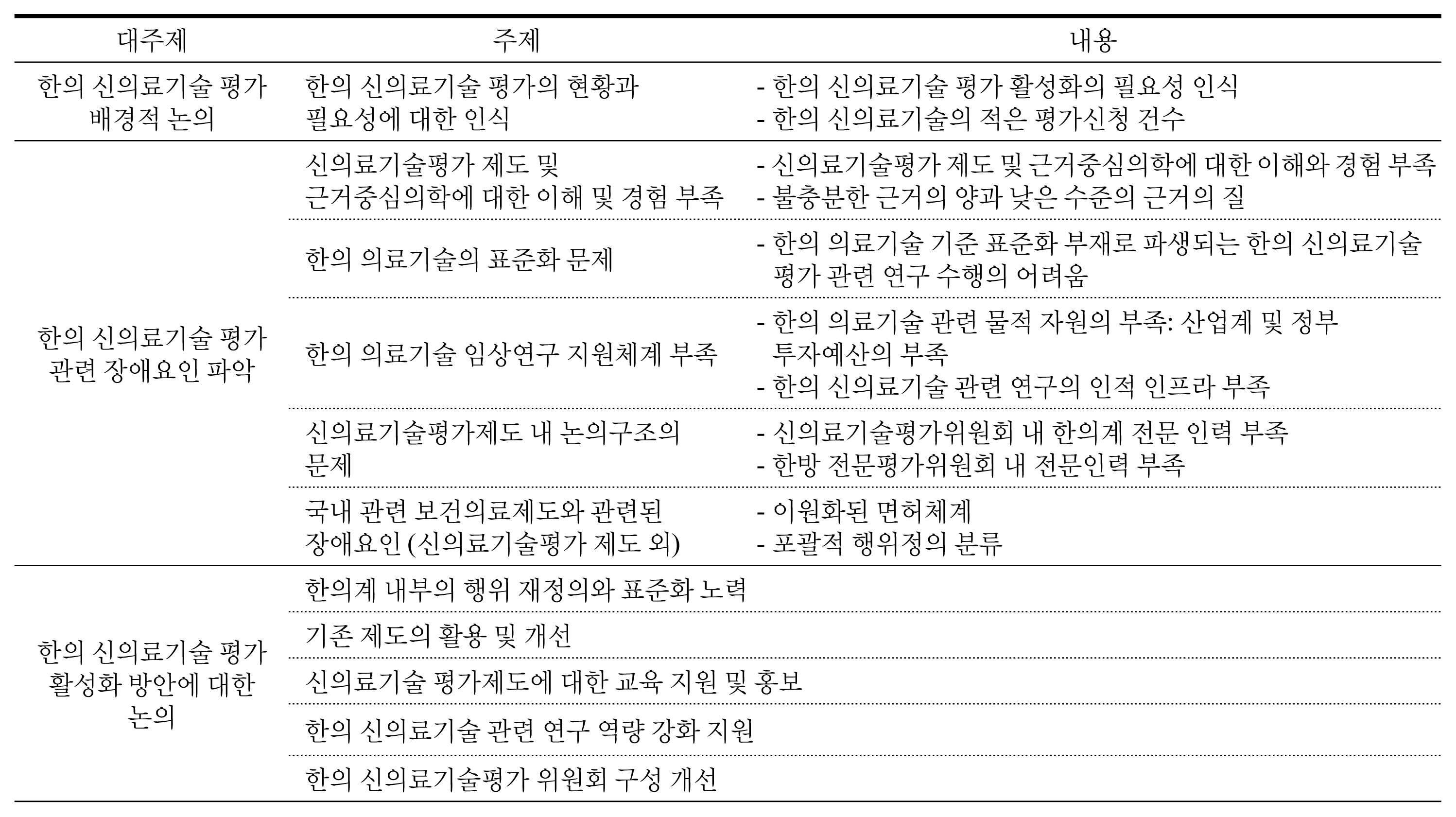
There were 31 cases in total except for duplications or reapplies falling into 3 main types. First, 19 of them were to try to enter a medical market and be covered by National Health Insurance. Eight cases were to apply western medicine technology as new health technology in Korean medicine area. The rest was 4 cases, which were totally not appropriate for the purpose of new health technology assessment system. According to the expert opinion, the obstacles of activation in new health technology assessment of Korean medicine were application of unstandardized technology, lack of understanding and experience, lack of clinical trial supporting system for Korean medicine, lack of committee members within the nHTA(new Health Technology Assessment) review board, ambiguous definition of medical practice and sharp conflict between western medicine and Korean medicine.
Conclusions
Several suggestions were derived. First of all, to activate Korean medicine in the nHTA system, the existing system should be used sufficiently, and multifaceted efforts are needed to upgrade the system, if necessary. Also, self-help efforts, Korean medicine clinical trial supporting system and increasing R&D investment, establishing extra-committee for Korean medicine in nHTA could be needed. Finally, long-term strategy for improving collaboration between Korean medicine and western medicine should be considered.