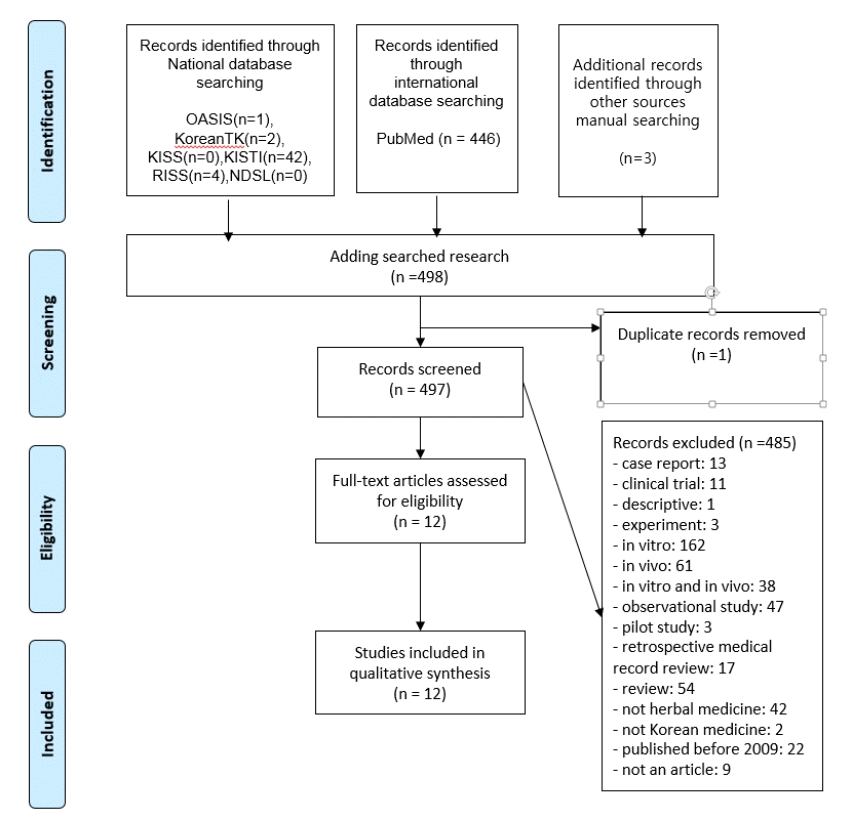
References
1. National Center Information Cancer[Internet] Goyang-si, Republic of Korea: Ministry of Health and Welfare; [cited 2019 Jan 25]. Available from :
https://www.cancer.go.kr.
2. Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–691.
3. Global Cancer Observatory[Internet] Lyon, France: International Agency for Research on Cancer; [cited 2019 Jan 25]. Available from:
http://gco.iarc.fr/today/home.
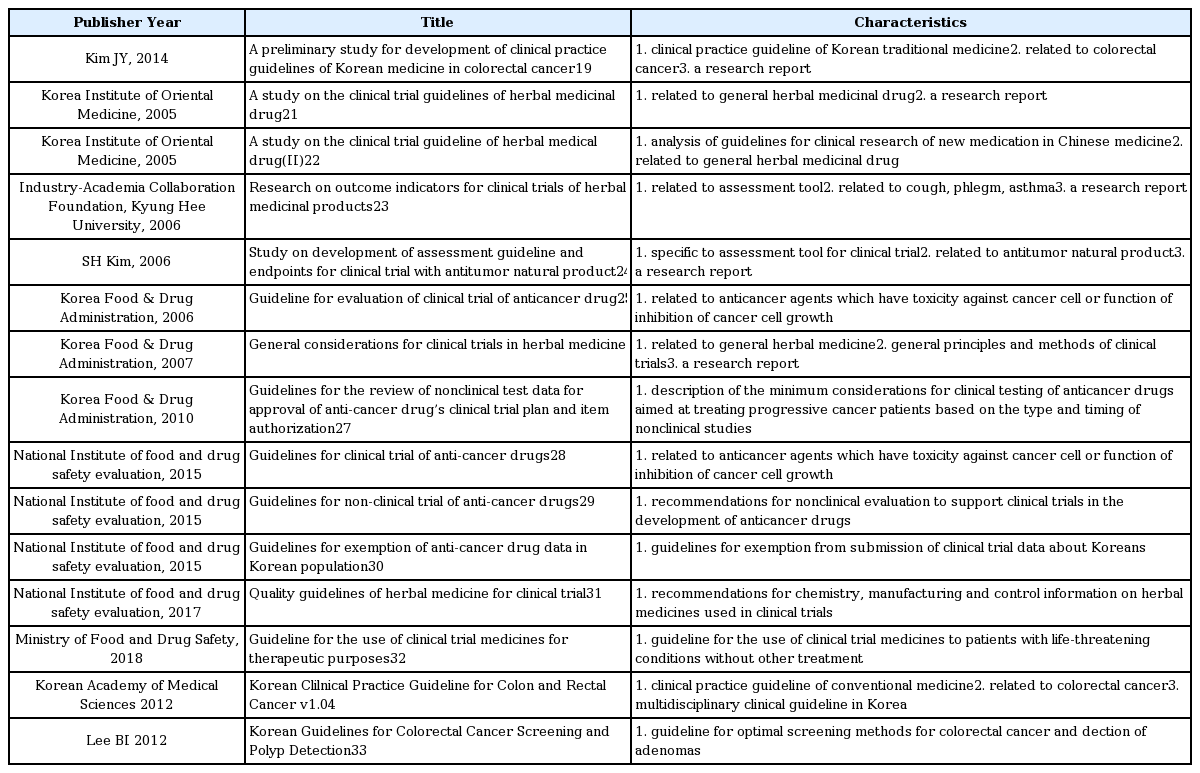
4. Committee of Korean Clinical Practice Guideline for Colon and Rectal Cancer. Korean Clilnical Practice Guideline for Colon and Rectal Cancer v1.0 Seoul: Korean Academy of Medical Sciences; 2012. p. 21–47.
6. Lee YJ. Management Behavior for Symptoms and Quality of Life on the Side Effects of Chemotherapy in Gastrointestinal Cancer Patients [master’s thesis] Suwon-si, Republic of Korea: Ajou university; 2012.
8. Lim SJ. Study on cancer patients presenting to the emergency department due to chemotherapy induced side effects [master’s thesis] Seoul, Republic of Korea: Seoul National University; 2013.
9. Lee SS, Park YJ, Han SH, Park JS. The Adverse Effects of Radiotherapy and Its Management in the Hospice and Palliative Care Patients. Korean journal of hospice and palliative care 2011;14(2):61–70.
10. Kim EH, Suh SR. A longitudinal path analysis of symptom, fatigue and quality of life in patients with colorectal cancer during chemotherapy. J Health Info Stat 2018;43(3):200–207.
11. Takegawa Y, Ikushima H, Ozaki K, Furutani S, Kawanaka T, Kudoh T, et al. Can Kampo therapy prolong the life of cancer patients? J Med Invest 2008;55(1–2):99–105.
12. Zhuang SR, Chen SL, Tsai JH, Huang CC, Wu TC, Liu WS, et al. Effec of citronellol and the Chinese medical herb complex on cellular immunity of cancer patients receiving chemotherapy/radiotherapy. Phytother Res 2009;23(6):785–790.
13. Kang YH, Hong MN, Han CW, Choi JY, Park SH, Kim SY. Review on Clinical Studies of Traditional Herbal Medicine and Acupuncture Treatments for Colorectal Cancer Patients. J Physiol & Pathol Korean Med 2016;30(4):219–28.
14. Han GJ, Seong S, Kim SS, Kim JS, Park JW. Analysis of Existing Guidelines and Randomized, Controlled, Clinical Trials for Development of [Guideline of Clinical Trial with Herbal Medicinal Product for gastric cancer]. J Korean Med 2017;38(3):124–142.
15. Luo Q, Asher GN. Complementary and alternative medicine use at a comprehensive cancer center. Integr Cancer Ther 2017;16:104–109.
16. Park BK, Wang JH, Cho JH, Son CG. Review of Randomized Controlled Trials using Herbal Remedies on Cancer Patients. J Korean Oriental Med 2010;31(5):12–32.
17. Oh HK, Lee JY, Ryu HS, Yoon SW. Review of Clinical cancer Research Methodology of Botanical Agents. J of Kor Traditional Oncology 2015;20(1):11–21.
18. Lee YM. Introduction to Evidence Based Medicine. Vascular Specialist International 2003;19(2):212–9.
19. Kim JY, Yoo HS, Cho JH. A Preliminary Study for Development of Clinical Practice Guidelines of Korean Medicine in Colorectal Cancer. Journal of Haehwa Medicine 2016;24(2):65–82.
20. Shin SG. Clinical trial status and prospect of domestic drug Seoul: Korea Health Industry Development Institute; 2006. p. 7–14.
21. Shin HG, Lee KG, Bae SH. A study on the clinical trial guidelines of herbal medicinal drug Seoul: Ministry of Food and Drug Safety; 2005. p. 1–174.
22. Shin HK, Kang JS, Kim YC, Lee KG, Yu YB, Bae SH. A Study on the clinical trial guideline of herbal medical drug(II) Seoul: Ministry of Food and Drug Safety; 2005. p. 1–538.
23. Ko SG, Jeong SK, Kwon DR, Seon SH, Ko HY, Jang BH, et al. Research on outcome indicators for clinical trials of herbal medicinal products Seoul: Ministry of Food and Drug Safety; 2006. p. 16–132.
24. Kim SH, Namgung MA, Chang YS, Jeong SK, Kim JS, Yoon SW, et al. Study on Development of Assessment Guideline and Endpoints for Clinical Trial with Antitumor Natural Products. Korean J Oriental Physiology & Pathology 2006;20(6):1678–1727.
25. Chung MA. Guideline on evaluation of clinical trial of anticancer drugs Seoul: The Pharmaceutical Society of Korea; 2006. p. 145.
26. Ministry of Food and Drug Safety. General considerations in clinical trials of herbal medicine preparations Seoul: Ministry of Food and Drug Safety; 2007.
27. Drug Evaluation Department Oncology and Antimicrobial Products Division. Guidelines for clinical testing of anti-cancer drugs and non-clinical test data for approval of items Cheongju: Korea Food & Drug Administration; 2010. p. 1–13.
28. Lee SH, Han ES, Kim SH, Yun KE, Eom JE, Park SR, et al. Guidelines for clinical trial of anti-cancer drugs Cheongju: National Institute of Food and Drug Safety Evaluation; 2015. p. 1–31.
29. Lee SH, Han ES, Kim SH, Yun KE, Eom JE, Park SR, et al. Guidelines for non-clinical trial of anti-cancer drugs Cheongju: National Institute of Food and Drug Safety Evaluation; 2015. p. 1–13.
30. Lee SH, Han ES, Kim SH, Yun KE, Park SR, Kim DH, et al. National Institute of Food and Drug Safety Evaluation. Guidelines for Exemption of Anticancer Drug Data in Bridging Study Cheongju: National Institute of Food and Drug Safety Evaluation; 2016. p. 1–26.
31. Kim DC, Park JY, Moon HJ, Kang IH, Kim JY, Kim HM, et al. Quality guidelines of herbal medicine for clinical trial Cheongju: National Institute of Food and Drug Safety Evaluation; 2017. p. 1–27.
32. Lee NH, Lee SD, Kim HS, Kim BS, Kang MS, Lee IS, et al. Guideline for the use of clinical trial medicines for therapeutic purposes Cheongju: Ministry of Food and Drug Safety; 2018. p. 1–47.
33. Lee BI, Hong SP, Kim SE, Kim SH, Kim HS, Hong SN, et al. Korean Guidelines for Colorectal Cancer Screening and Polyp Detection. Korean J Gastroenterol 2012;59(2):65–84.
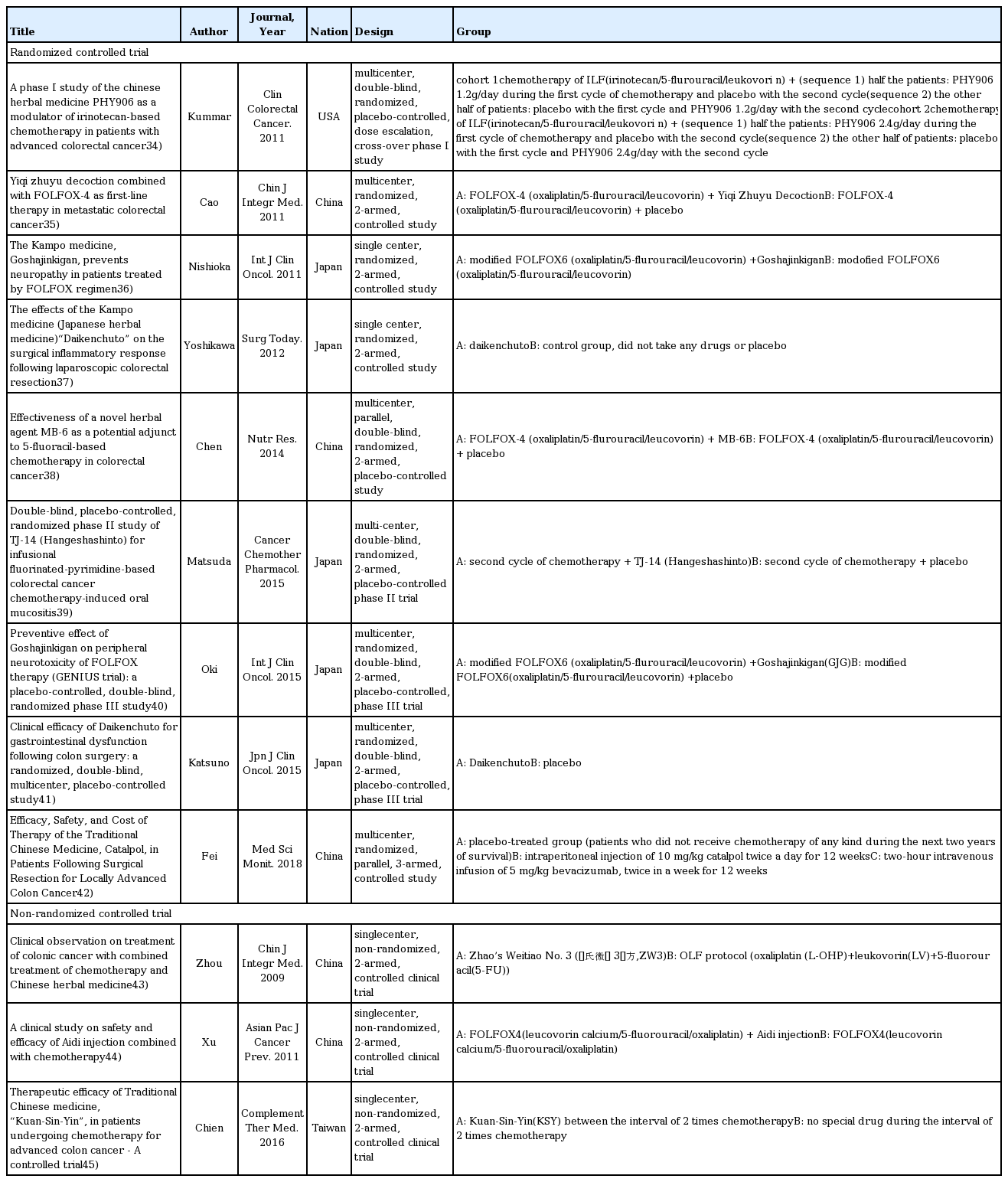
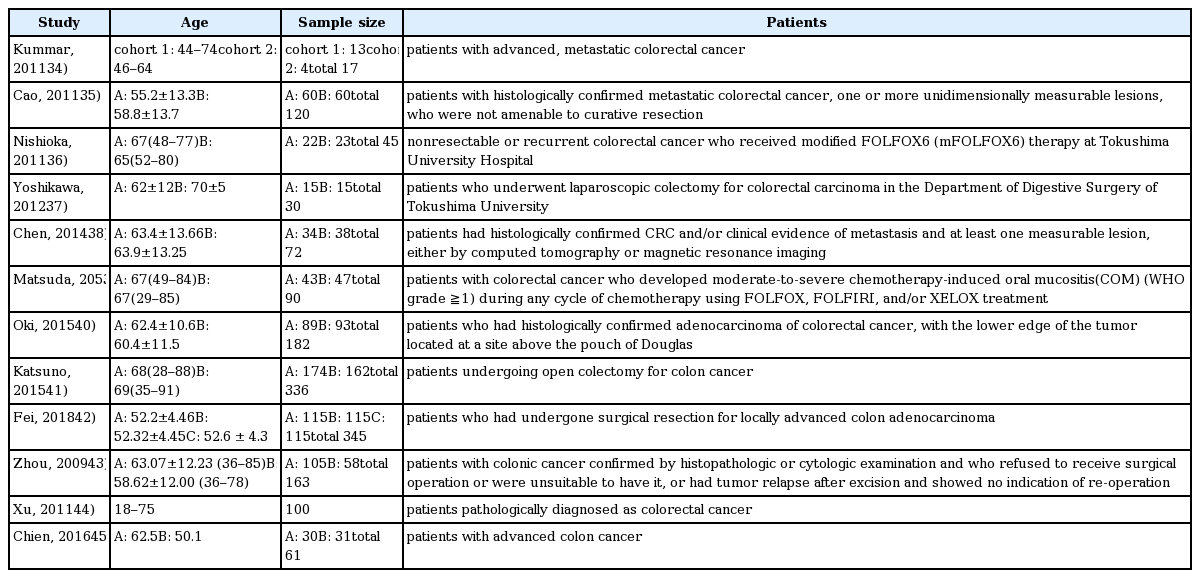
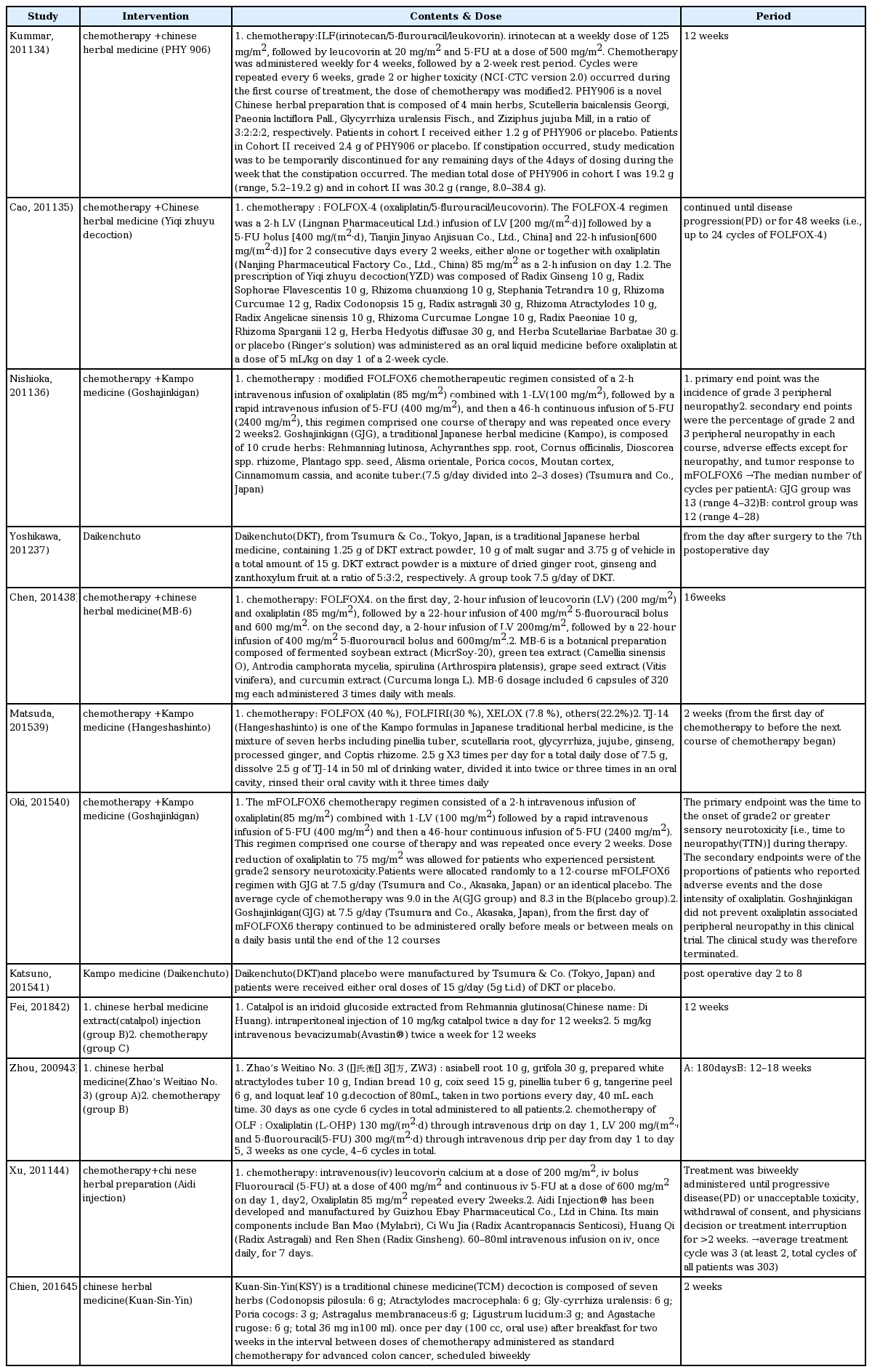
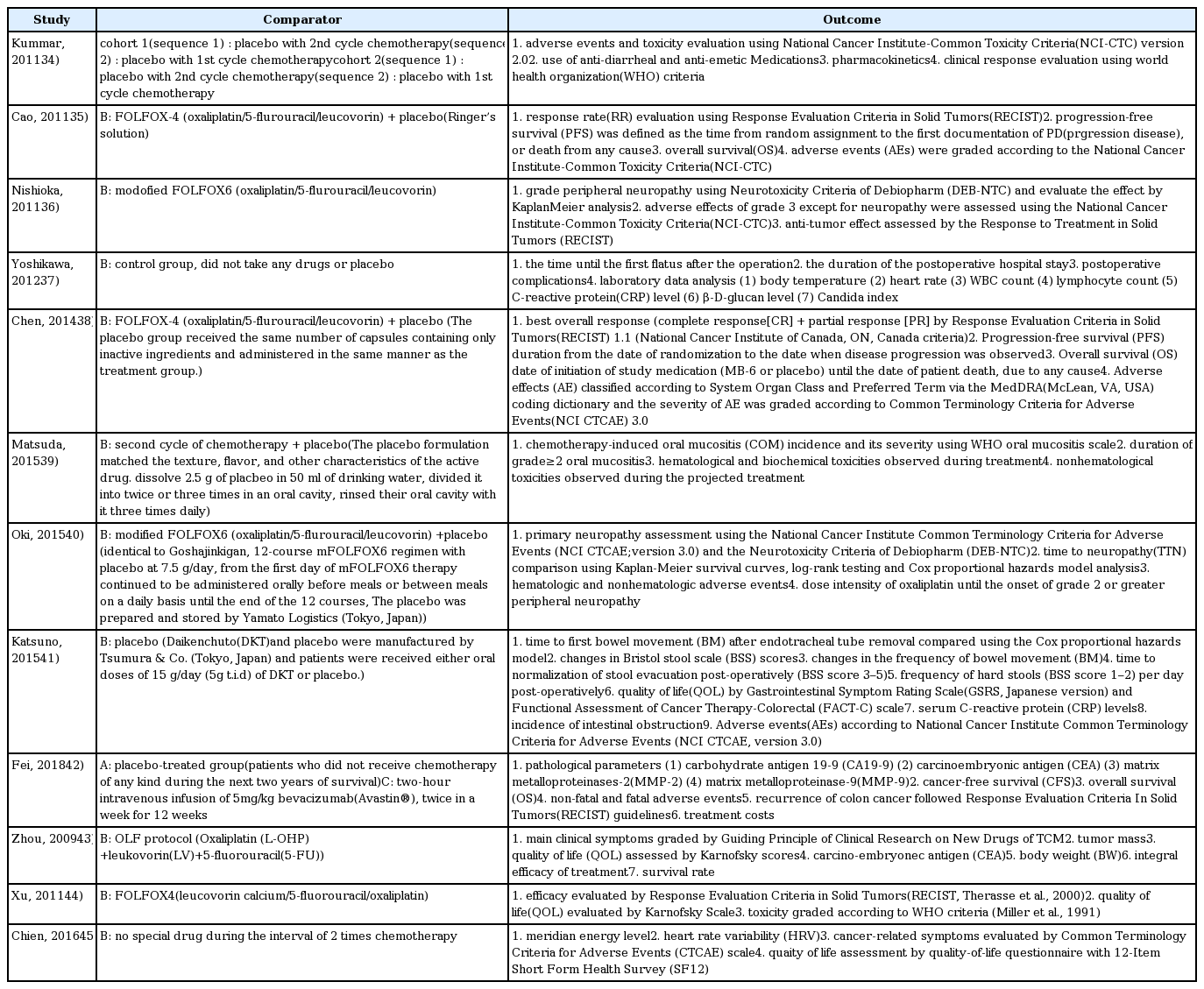
34. Kummar S, Copur MS, Rose M, Wadler S, Stephenson J, O’Rourke M, Brenckman W, Tilton R, Liu SH, Jiang Z, Su T, Cheng YC, Chu E. A phase I study of the chinese herbal medicine PHY906 as a modulator of irinotecan-based chemotherapy in patients with advanced colorectal cancer. Clin Colorectal Cancer 2011;10(2):85–96.
35. Cao B, Li ST, Li Z, Deng WL. Yiqi zhuyu decoction combined with FOLFOX-4 as first-line therapy in metastatic colorectal cancer. Chin J Integr Med 2011;17(8):593–9.
36. Nishioka M, Shimada M, Kurita N, Iwata T, Morimoto S, Yoshikawa K, Higashijima J, Miyatani T, Kono T. The Kampo medicine, Goshajinkigan, prevents neuropathy in patients treated by FOLFOX regimen. Int J Clin Oncol 2011;16(4):322–7.
37. Yoshikawa K, Shimada M, Nishioka M, Kurita N, Iwata T, Morimoto S, et al. The effects of the Kampo medicine (Japanese herbal medicine) “Daikenchuto” on the surgical inflammatory response following laparoscopic colorectal resection. Surg Today 2012;42(7):646–51.
38. Chen WT, Yang TS, Chen HC, Chen HH, Chiang HC, Lin TC, et al. Effectiveness of a novel herbal agent MB-6 as a potential adjunct to 5-fluoracil-based chemotherapy in colorectal cancer. Nutr Res 2014;34(7):585–94.
39. Matsuda C, Munemoto Y, Mishima H, Nagata N, Oshiro M, Kataoka M, et al. Double-blind, placebo-controlled, randomized phase II study of TJ-14 (Hangeshashinto) for infusional fluorinated-pyrimidine-based colorectal cancer chemotherapy-induced oral mucositis. Cancer Chemother Pharmacol 2015;76(1):97–103.
40. Oki E, Emi Y, Kojima H, Higashijima J, Kato T, Miyake Y, et al. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol 2015;20(4):767–75.
41. Katsuno H, Maeda K, Kaiho T, Kunieda K, Funahashi K, Sakamoto J, et al. Clinical efficacy of Daikenchuto for gastrointestinal dysfunction following colon surgery: a randomized, double-blind, multicenter, placebo-controlled study. Jpn J Clin Oncol 2015;45(7):650–6.
42. Fei B, Dai W, Zhao S. Efficacy, Safety, and Cost of Therapy of the Traditional Chinese Medicine, Catalpol, in Patients Following Surgical Resection for Locally Advanced Colon Cancer. Med Sci Monit 2018;24:3184–92.
43. Zhou LY, Shan ZZ, You JL. Clinical observation on treatment of colonic cancer with combined treatment of chemotherapy and Chinese herbal medicine. Chin J Integr Med 2009;15(2):107–11.
44. Xu HX, Huang XE, Li Y, Li CG, Tang JH. A clinical study on safety and efficacy of Aidi injection combined with chemotherapy. Asian Pac J Cancer Prev 2011;12(9):2233–6.
45. Chien TJ, Liu CY, Lu RH, Kuo CW, Lin YC, Hsu CH. Therapeutic efficacy of Traditional Chinese medicine, “Kuan-Sin-Yin”, in patients undergoing chemotherapy for advanced colon cancer - A controlled trial. Complement Ther Med 2016;29:204–12.
46. Cheon CH, Park SJ, Jang BH, Shin YC, Ko SG. The Analysis of usage of Symptom Differentiation in Clinical Trials in Korean Medicine for Cancer Patients. spkom 2015;19(1):61–70.
47. Lyseng-Williamson KA, Fenton C. Docetaxel: a review of its use in metastatic breast cancer. Drugs 2005;65(17):2513–31.
48. Jeong TY, Lee YW, Cho JG, Yoo HS. Analysis of Clinical Characteristics for 899 Cancer Patients Treated at an Oriental Hospital. Korean J Orient Int Med 2010;31(1):102–12.
49. Yoshikawa K, Shimada M, Wakabayashi G, Ishida K, Kaiho T, Kitagawa Y, et al. Effect of Daikenchuto, a Traditional Japanese Herbal Medicine, after Total Gastrectomy for Gastric Cancer: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase II Trial. J Am Coll Surg 2015;221(2):571–8.
50. Krishnan AV, Goldstein D, Friedlander M, Kiernan MC. Oxaliplatin-induced neurotoxicity and the development of neuropathy. Muscle Nerve 2015;32:51–60.
51. Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, et al. Mucositis study section of the multinational association for supportive care in cancer; international society for oral oncology. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004;100:2026–46.
52. Brierley J, Gospodarowicz M, O’Sullivan B. The principles of cancer staging. E cancer medical science 2016. 10p. ed61.
53. Kim JB, An KS. A Comparative Study between East and West Medicine on the Colorectal Cancer. Journal of physiology & pathology in Korean medicine 1995;10:89–127.
54. Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR. How quality of life data contribute to our understanding of cancer patients’ experiences? A study of patients with lung cancer. Quality of Life Research 2003;12:157–166.
55. Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR. Quality of life in patients with lung cancer: as an important prognostic factor. Lung Cancer 2001;31:233–40.
56. Lee NH, Cho JH, Son CG, Yoo HS, Lee YW, Yoon DH, et al. Analysis of Studies on Quality of Life according to Cancer Types and Symptoms. Korean J Orient Int Med 2006;27(3):555–60.
57. Lee EH, Park HB, Kim MW, Kang SH, Lee HJ, Lee WH, et al. Analyses of the Studies on Cancer-Related Quality of Life Published in Korea. Radiat Oncol J 2002;29(4):359–66.
58. Kim HY. Tumor response evaluation(WHO vs. RECIST) and problems. J Clin Cancer Res 2007;1(1):1–6.
59. Therasse P1, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada., J Natl Cancer Inst 2000;92(3):205–16.
60. Kim HY. Tumor response assessment (WHO vs. RECIST) and problems. J Clin Cancer Res 2007;1(2):1–6.
61. Ministry of Food and Drug Safety. Evaluation of the safety information of the client requesting pharmaceutical clinical trials and considerations for reporting 2017. p. 8.