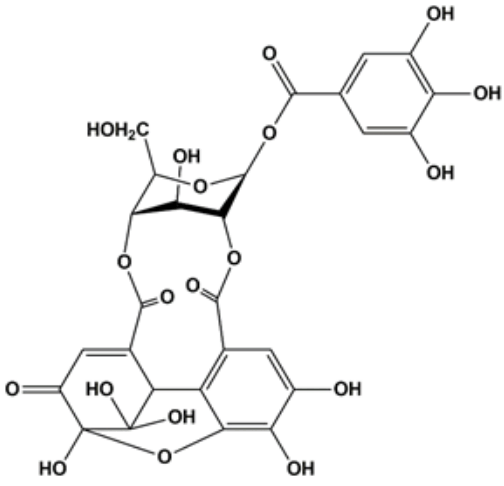
Protective effect of furosin isolated from Euphorbia helioscopia against glutamate-induced HT22 cell death
Article information
Abstract
Objectives
In the brain, glutamate is the most important excitable neurotransmitter in physiological and pathological conditions. However, the high level of glutamate induces neuronal cell death due to exitotoxicity and oxidative stress. The present study investigated to evaluate a possible neuroprotective effect of furosin isolated from Euphorbia helioscopia against glutamate-induced HT22 cell death.
Methods
Furosin was isolated from methanol extract of Euphorbia helioscopia and examined whether it protects glutamate-induced neuronal cell death. The cell viability was determined using Ez-Cytox assay. Anti-oxidative effect of furosin was determined by DPPH scavenging activities, and the levels of intracellular reactive oxygen species (ROS) were determined by the fluorescent intensity after staining the cells with H2DCFDA. To evaluate apoptotic cell death, we performed nuclear staining and image-based cytometeric analysis.
Results
The cell viability was significantly increased by treatement with furosin compared with the treatment with glutamate. Furosin showed a strong DPPH radical scavenging activity (EC50=1.83 μM) and prevented the accumulation of intra cellular ROS. Finally, the presence of 50 and 100 μM furosin significantly the percentage of apoptotic cells compared with glutamate treatment.
Conclusion
The present study found that furosin is a potent neuroprotectant against glutamate-induced oxidative stress through inhibition of apoptotic cell death induced by glutamate. Therefore, the present study suggests that furosin as a bioactive compound of E. helioscopia can be a useful source to develop a drug for the treatment of neurodegenerative diseases and acute brain injuries.
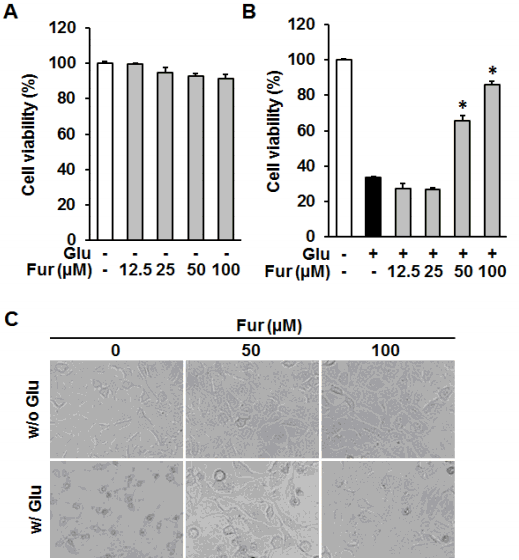
Furosin inhibited glutamate-induced HT22 cell death. HT22 cells were treated with the indicated concentrations of furosin in the presence or absence of 5 mM glutamate. (A and B) To determine the self toxicity and protective effect of furosin, the cell viability was determined using Ez-Cytox assay kit after the exposure to glutamate for 24 h. Bars denote the percentage of cell viability compared with non-treated groups (mean±S.E.M., *p<0.05 compared with glutamate-treated group). (C)Morphological changes were observed under the phase contrast microscope.
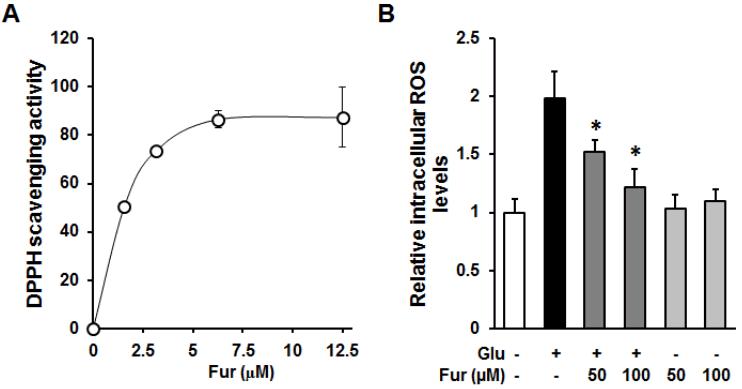
Furosin prevented intracellular ROS accumulation through their antioxidant capacity. (A)The indicated concentrations of furosin was incubated with DPPH radical reagent for 30 min and obtained using microplate reader at 550 nm. (B)HT22 cells were treated with 50 or 100 uM furosin in the presence (dark gray) or absence (gray) of 5 mM glutamate for 8 h and stained with H2DCFDA, a fluorescent indicator of ROS. The fluorescent intensity of DCF was measured using a fluorescent microplate reader. Bars denote the fold increases of intracellular ROS level compared with non-treated groups (mean ±S.E.M., *<0.05 compared with glutamate-treated group).
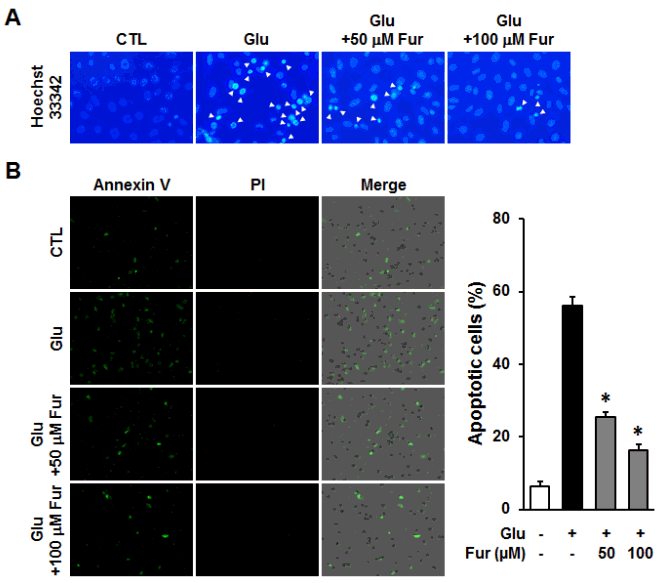
Furosin prevented glutamate-induced apoptotic HT22 cell death. HT22 cells were incubated with glutamate in the presence or absence of furosin for 12 h. (A) Chromatin condensation was determined using Hoechst33342 and observed under a fluorescent microscope. (B) The number of apoptotic cells were analyzed using Tali-image based cytometric analysis. The proportion of apoptotic cells were represented by the percentage of Annexin V-positive cells (mean±S.E.M., * <0.05 compared with glutamate-treated group).