References
1. Ministry of Health and Welfare. National Cancer Registration Statistics Reference 2014 2016. 12. 20.
3. Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Synopsis on Clinical Practice Guideline of Gastric Cancer in Korea: An Evidence-Based Approach. Korean J Gastroenterol 2014;63(2):66–81.
4. Cheung DY, Kim JK. Perspectives of the Stomach Cancer Treatment: The Introduction of Molecular Targeted Therapy and the Hope for Cure. Korean J Gastroenterol 2013;61(3):117–27.
5. Jang SB, et al. A Study on Complementary Alternative Therapy Used by Cancer Patients in Korea. Korean J Adult Nurs 2006;18(1):92–101.
6. Lee Ei, Shin YC, Lee JH, Kim SD, Kim HJ, Cho MS. Use of Complementary and Alternative Medicine in Cancer Patients at 7 General Hospitals in Seoul. J Korea Public Health Assoc 2002;28(3):225–38.
7. Ha TH, Seong S, Lee DH, Kim SS. Improved Case of Recurred and Metastatic Ascending Colon Cancer by Combination of Oriental Medical Therapy and FOLFIRI Chemotherapy. Korean J Oriental Physiology & Pathology 2013;26(1):148–51.
8. Cheon SH, Kim KS, Kim S, Jung HS, Choi WC, Eo WK. Efficacy and Safety of Rhus verniciflua Stokes Extracts in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. Forsch Komplementmed 2011;18(2):77–83.
9. Kim BJ, Moon G. An Outlook of the Oriental and Western Medical Diagnosis and Treatment on Gastric Cancer. KOMS 1996;17(2):100–16.
10. Hwang CY. Bibliographic Study on the Therapy of Gastric Cancer by Intergrated Oriental and Western Medicine. The Journal of Wonkwang Oriental Medicine 1997;7(1):10–8.
11. Jung JH, Seo JC, Kwak MA, Sohn KC. Literature Review on Traditional Chinese Medicine Treatment of Gastric Cancer. J Int Korean Med 2014;35(3):332–42.
12. Sim BS, Choi SH. A Literatural Study on Pattern Identification Type of Stomach Cancer. J of Oriental Medical Pathology 1993;8:295–303.
13. Nationwide Medical College of Oriental Gastroenterology Medicine. Oriental Gastroenterology Seoul: Koonja Publisher Co; 2008. p. 126–36.
14. Korea Health Industry Development Institute. Clinical trial status and prospect of domestic drug Seoul: Korea Health Industry Development Institute; 2006. p. 7–14.
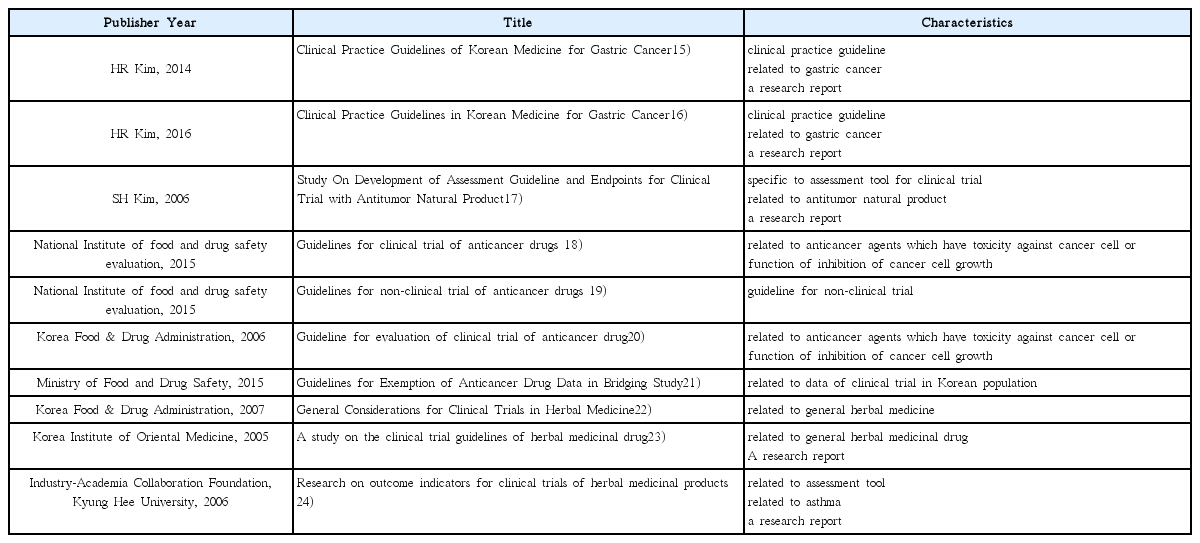
15. Kim HR, Jeong HR, Baek DG, Won JH, Moon G. Clinical Practice Guidelines of Korean Medicine for Gastric Cancer. Journal of Korean traditional oncology 2014;19(1):1–24.
16. Kim HR, Yoo HS, Beak DG, Park IH, Jang CY, Kim HY. Clinical Practice Guidelines in Korean Medicine for Gastric Cancer. Korean J Orient Int Med 2016;37(1):25–46.
17. Kim SH, Namgung MA, Chang YS, Jeong SK, Kim JS, Yoon SW, et al. Study on Development of Assessment Guideline and Endpoints for Clinical Trial with Antitumor Natural Products. Korean J Oriental Physiology & Pathology 2006;20(6):1678–1727.
18. National Institute of Food and Drug Safety Evaluation. Guidelines for clinical trial of anticancer drugs 2015. p. 12.
19. National Institute of Food and Drug Safety Evaluation. Guidelines for non-clinical trial of anticancer drugs 2015. p. 12.
20. Ministry of Food and Drug Safety. Guideline for evaluation of clinical trial of anticancer drugs
21. National Institute of Food and Drug Safety Evaluation. Guidelines for Exemption of Anticancer Drug Data in Bridging Study 2015. 07
22. Ministry of Food and Drug Safety. General considerations in clinical trials of herbal medicine preparations Seoul: Ministry of Food and Drug Safety; 2007.
23. Korean Institution of Oriental Medicine. A study on the clinical trial guidelines of herbal medicinal drug Seoul: Ministry of Food and Drug Safety; 2004.
24. Industry-Academia Collaboration Foundation, Kyung Hee University. Research on outcome indicators for clinical trials of herbal medicinal products Seoul: Ministry of Food and Drug Safety; 2006.
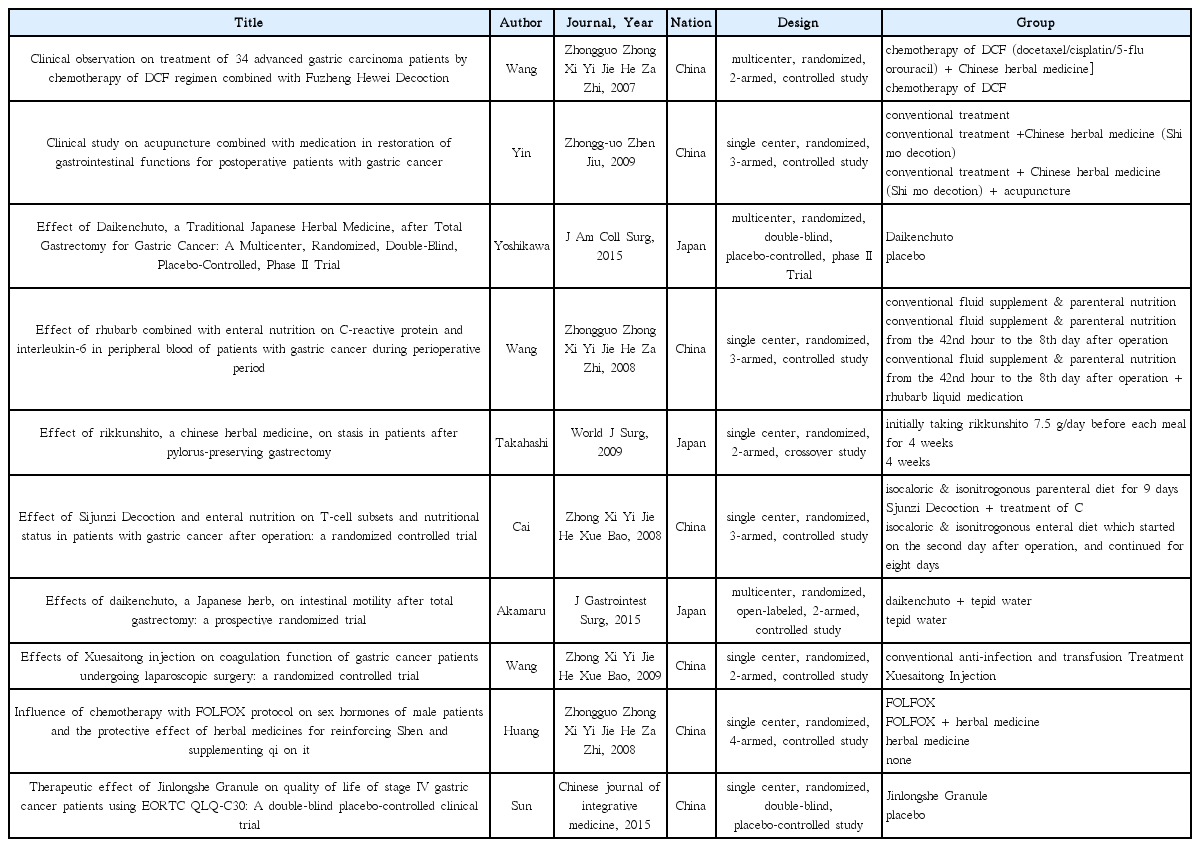
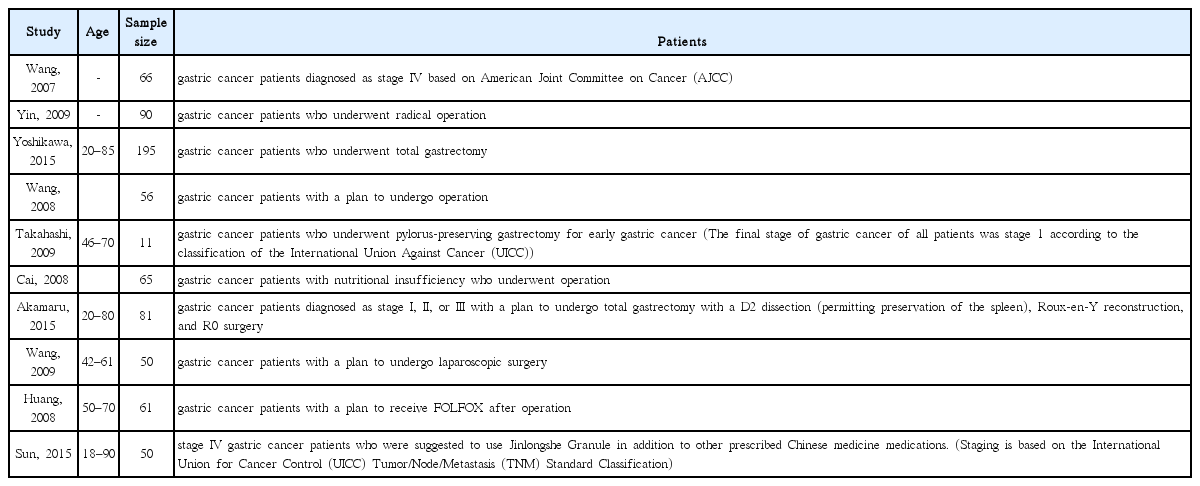
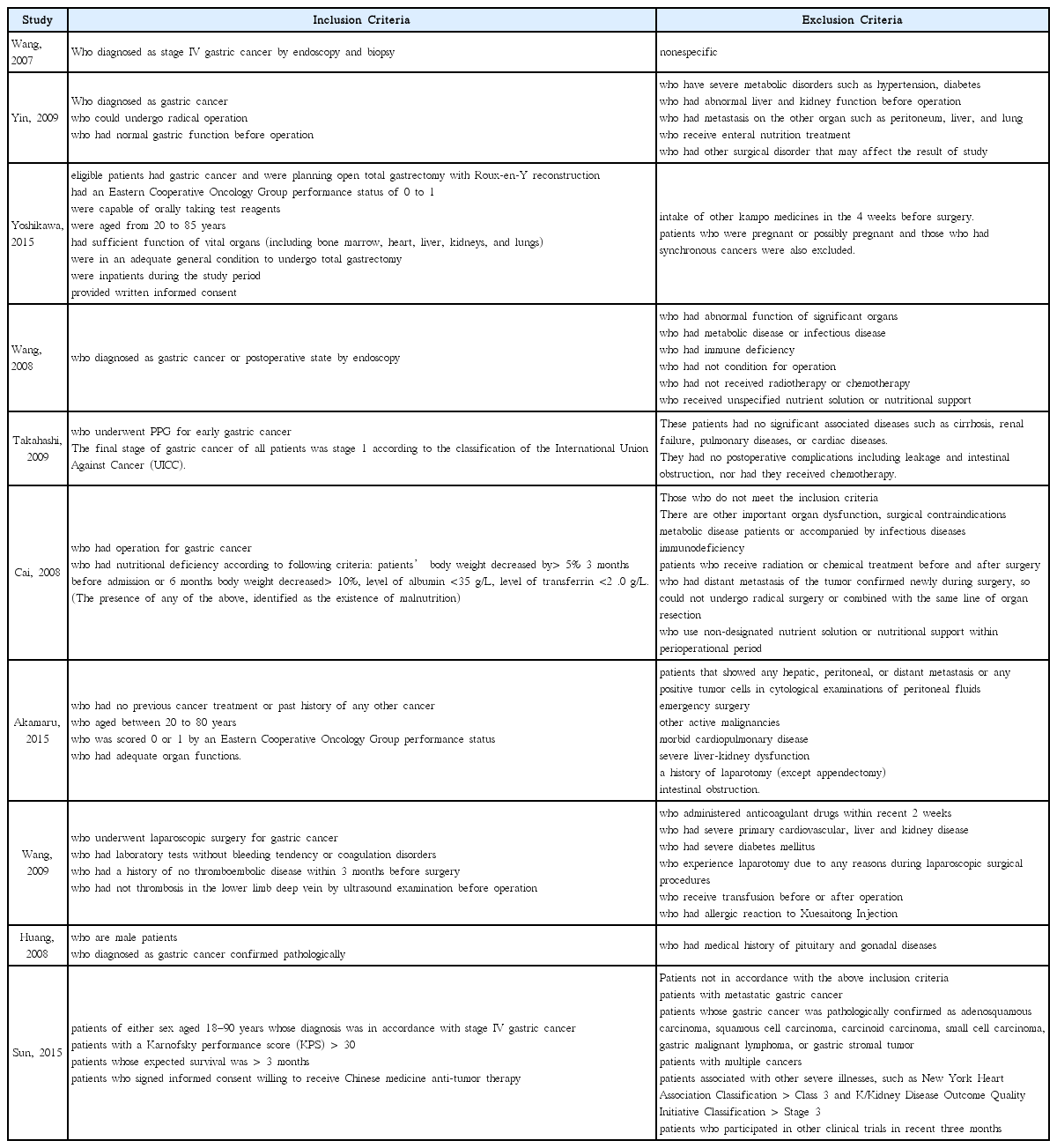
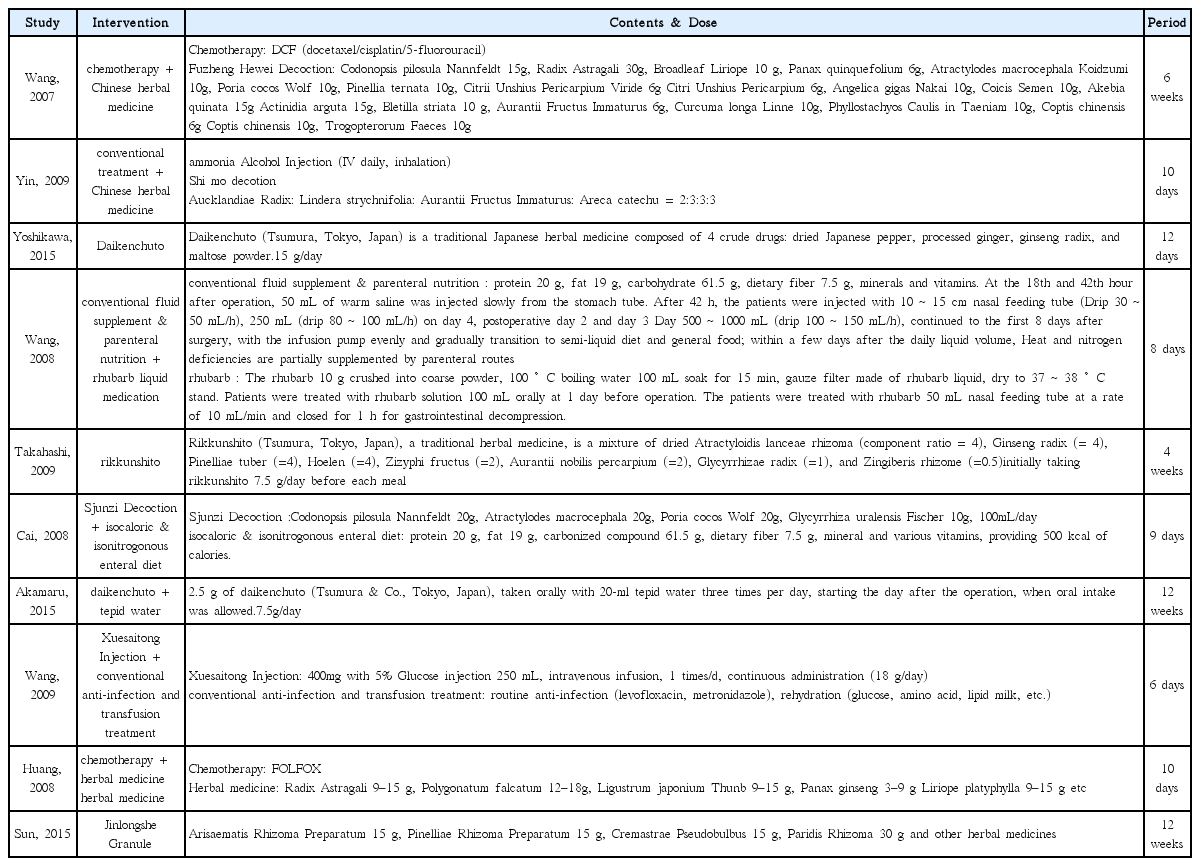
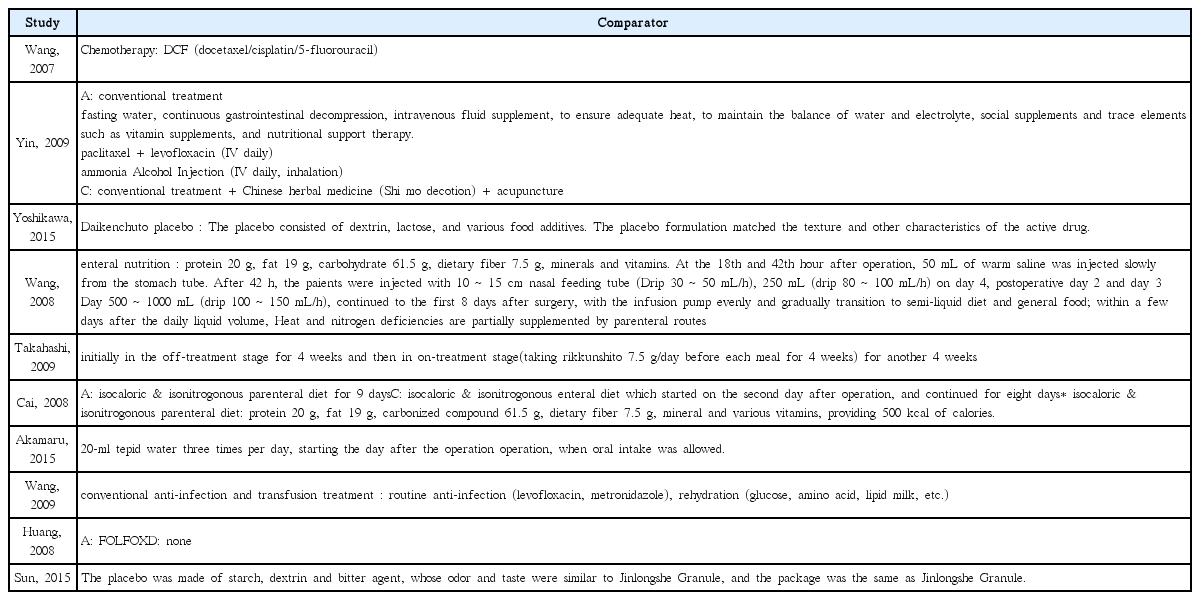
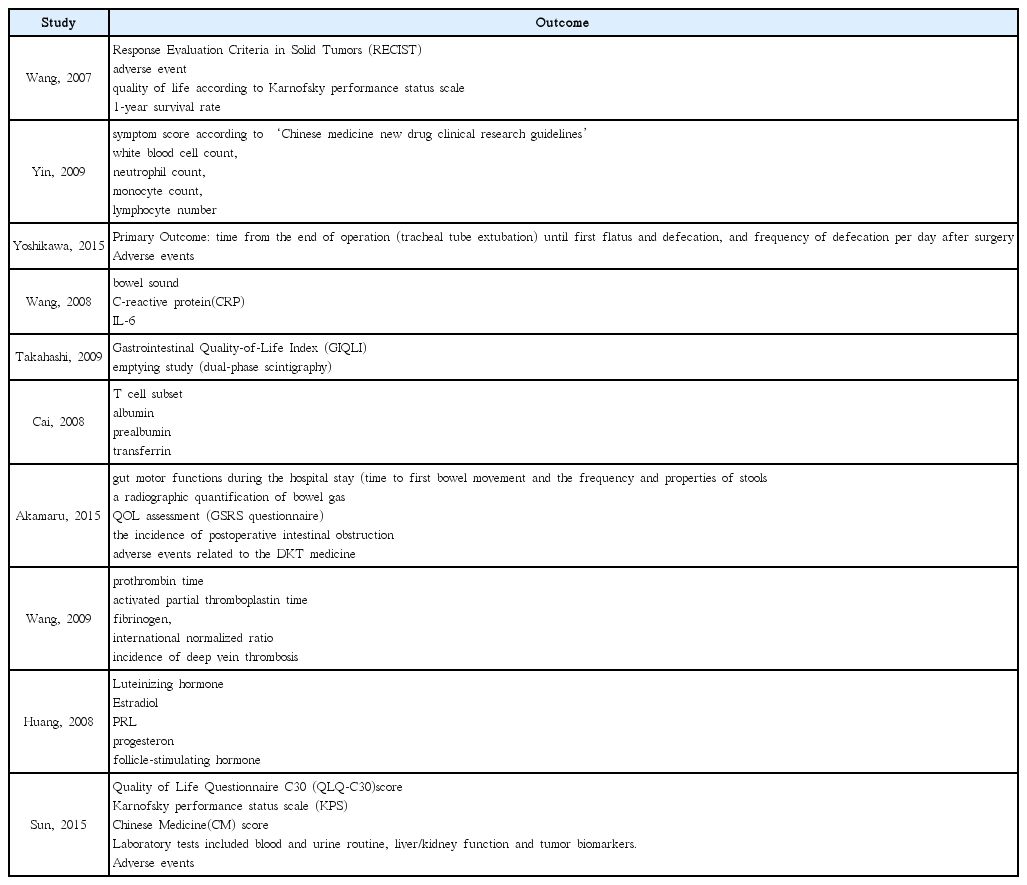
25. Yoshikawa K, Shimada M, Wakabayashi G, Ishida K, Kaiho T, Kitagawa Y, et al. Effect of Daikenchuto, a Traditional Japanese Herbal Medicine, after Total Gastrectomy for Gastric Cancer: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase II Trial. J Am Coll Surg 2015;221(2):571–8.
26. Takahashi T, Endo S, Nakajima K, Souma Y, Nishida T. Effect of rikkunshito, a chinese herbal medicine, on stasis in patients after pylorus-preserving gastrectomy. World J Surg 2009;33(2):296–302.
27. Akamaru Y, Takahashi T, Nishida T, Omori T, Nishikawa K, Mikata S, et al. Effects of daikenchuto, a Japanese herb, on intestinal motility after total gastrectomy: a prospective randomized trial. J Gastrointest Surg 2015;19(3):467–72.
28. Wang HZ, Wang HB, Gao H. Clinical observation on treatment of 34 advanced gastric carcinoma patients by chemotherapy of DCF regimen combined with Fuzheng Hewei Decoction. Zhongguo Zhong Xi Yi Jie He Za Zhi 2007;27(10):927–9.
29. Yin SH, Du YQ, Liu B. Clinical study on acupuncture combined with medication in restoration of gastrointestinal functions for postoperative patients with gastric cancer. Zhongguo Zhen Jiu 2009;29(6):459–62.
30. Wang H, Guo LJ, Wu B. Effect of rhubarb combined with enteral nutrition on C-reactive protein and interleukin-6 in peripheral blood of patients with gastric cancer during perioperative period. Zhongguo Zhong Xi Yi Jie He Za Zhi 2008;28(2):101–4.
31. Cai J, Wang H, Zhou S, Wu B, Song HR, Xuan ZR. Effect of Sijunzi Decoction and enteral nutrition on T-cell subsets and nutritional status in patients with gastric cancer after operation: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 2008;6(1):37–40.
32. Wang Y, Wang YT, Lin YH, Zou ZD. Effects of Xuesaitong injection on coagulation function of gastric cancer patients undergoing laparoscopic surgery: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 2009;7(6):514–7.
33. Huang CX, Chen LZ, Zhao JG. Influence of chemotherapy with FOLFOX protocol on sex hormones of male patients and the protective effect of herbal medicines for reinforcing Shen and supplementing qi on it. Zhongguo Zhong Xi Yi Jie He Za Zhi 2008;28(11):986–9.
34. Sun DZ, Jiao JP, Zhang X, Xu JY, Ye M, Xiu LJ, et al. Therapeutic effect of Jinlongshe Granule on quality of life of stage IV gastric cancer patients using EORTC QLQ-C30: A double-blind placebo-controlled clinical trial. Chin J Integr Med 2015;21(8):579–86.
35. Cheon CH, Park SJ, Jang BH, Shin YC, Ko SG. The Analysis of usage of Symptom Differentiation in Clinical Trials in Korean Medicine for Cancer Patients. spkom 2015;19(1):61–70.
36. Lyseng-Williamson KA, Fenton C. Docetaxel: a review of its use in metastatic breast cancer. Drugs 2005;65(17):2513–31.
37. Jeong TY, Lee YW, Cho JG, Yoo HS. Analysis of Clinical Characteristics for 899 Cancer Patients Treated at an Oriental Hospital. Korean J Orient Int Med 2010;31(1):102–12.
38. Lin H. Guidelines of Diagnosis and Therapy in Oncology with Traditional Chinese Medicine Beijing: Renminweisheng Press; 2014. p. 306–38.
39. Lee JI, Shim KY, Kim HY, Choi SY, Bang DG, Cho KS. Clinical Study of the Efficacy of Combined Western-Oriental Medicine for Gastric Cancer and Hepatocellular Carcinoma. J of Kor Traditional Oncology 2001;7(1):117–129.
40. Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Synopsis on Clinical Practice Guideline of Gastric Cancer in Korea: An Evidence-Based Approach. Korean J Gastroenterol 2014;63(2):66–81.
41. Kong KH, Ha J, Baek TH. One Case on Diagnosis and Treatment Based on an Overall Analysis of Signs and Symtoms of Stomach Cancer stage IV. Korean J Orient Int Med 2000;21(5):897–902.
42. Sim BS, Choi SH. A Literatural Study on Pattern Identification Type of Stomach Cancer. J of Oriental Medical Pathology 1993;8:295–303.
43. Hwang SY, Ahn SH, Keum KS. Proposal on Supplementation to Oriental Medical Policy for the Revitalization of Oriental Medical Therapy for Stomach Cancer. Korean J Oriental Physiology & Pathology 2009;23(3):528–33.
44. Nationwide Medical College of Oriental Gastroenterology Medicine. Oriental Gastroenterology Seoul: Koonja Publisher Co; 2008. p. 126–36.
45. Lee NH, Cho JH, Son CG, Yoo HS, Lee YW, Yoon DH, et al. Analysis of Studies on Quality of Life according to Cancer Types and Symptoms. Korean J Orient Int Med 2006;27(3):555–60.
46. Lee EH, Park HB, Kim MW, Kang SH, Lee HJ, Lee WH, et al. Analyses of the Studies on Cancer-Related Quality of Life Published in Korea. Radiat Oncol J 2002;29(4):359–66.