Effect of Gamiondam-tang (GMODT), a Polyherbal Formula on the Pharmacokinetics Profiles of Tamoxifen in Male SD Rats
Article information
Abstract
Objectives
The effects of Gamiondam-tang (GMODT) co-administration within 5min on the pharmacokinetics (PK) of tamoxifen were observed as a process of the comprehensive and integrative medicine, combination therapy of tamoxifen with GMODT to achieve synergic pharmacodynamics and reduce toxicity on the breast cancer.
Methods
After 50mg/kg of tamoxifen treatment, GMODT 100mg/kg was administered within 5min. The plasma were collected at 30 min before administration, 30 min, 1, 2, 3, 4, 6, 8 and 24 hrs after end of GMODT treatment, and plasma concentrations of tamoxifen were analyzed using LC-MS/MS methods. PK parameters of tamoxifen (Tmax, Cmax, AUC, t1/2 and MRTinf) were analysis as compared with tamoxifen single administered rats using noncompartmental pharmacokinetics data analyzer programs.
Results
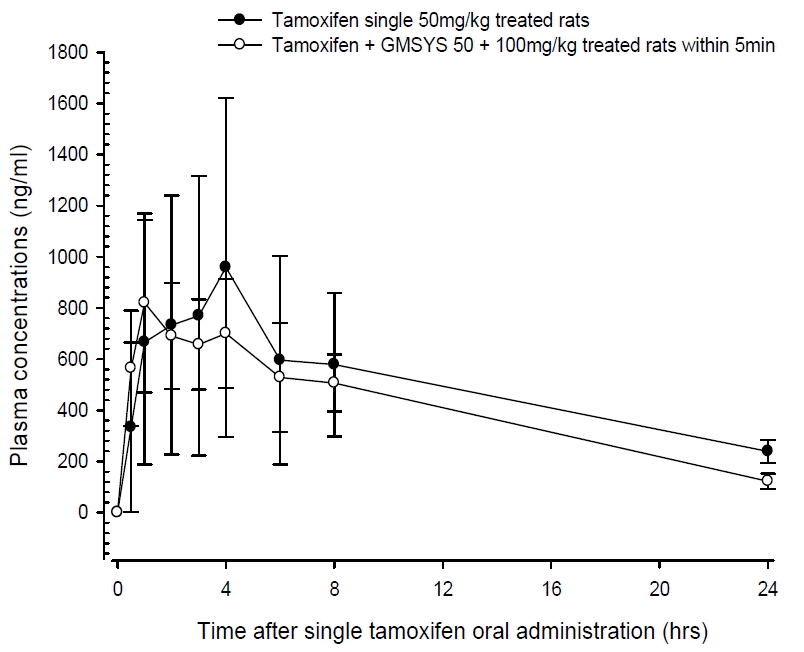
Co-administration with GMODT induced increased trends of plasma tamoxifen concentrations to 1hr after end of administration, and then showed decreased trends of plasma tamoxifen concentrations, and especially significant (p<0.05) increases of plasma tamoxifen concentrations were demonstrated at 0.5hr after end of co-administration with GMODT and also related significant (p<0.05) decreases of AUC0-inf and MRTinf as compared with tamoxifen single formula treated rats, at dosage levels of tamoxifen 10 mg/kg and GMODT 100 mg/kg within 5 min, in this experiment.
Conclusion
Based on the results of the present study, it is considered that single co-administration GMODT within 5min significantly inhibited the oral bioavailability of tamoxifen through variable influences on the absorption and excretion of tamoxifen, can be influenced on the toxicity or pharmacodynamic of tamoxifen.
Introduction
Tamoxifen (NolvadexTM) is a nonsteroidal estrogen agonist-antagonist antineoplastic agent has been used for breast cancer1). It is the usual endocrine (anti-estrogen) therapy for hormone receptor-positive breast cancer in pre-menopausal women, and is also a standard in post-menopausal women although aromatase inhibitors are also frequently used in that setting2,3). In addition, tamoxifen also used to treat infertility in women with anovulatory disorders4,5) and prevention for gynecomastia6,7) and bipolar disorder8,9), as anti-angiogenesis10), control of gene expression11), and treat Riedel thyroiditis12,13) and Albright’s syndrome14,15). Tamoxifen is contraindicated, when used in women with ductal carcinoma in situ and women at high risk for breast cancer, concurrent anticoagulant therapy with a warfarin derivative16,17), and be used with caution in patients with leukopenia or thrombocytopenia18,19) and pregnant20–22). Hot flashes, vaginal discharge, menstrual irregularities and weight loss are common side effects related with tamoxifen treatment20,23,24).
As results of combination therapies with other drugs to improve the side effects of tamoxifen or to achieve synergic effects, various drug-drug interactions of tamoxifen have been evaluated; because tamoxifen was metabolized by a substrate of CYP3A, 2C9, 2D625,26), it interacted with various drugs, namely, combinations containing any of the following medications, depending on the amount present, may also interact with aminoglutethimide27), anticoagulants28,29), bromocriptine30), letrozole31), medroxyprogesterone32), phenobarbital33), rifampin34), and cyclosporine, erythromycin, diltiazem, erythromycin and nifedipine35–37). However, interactions with herbal products have not been established except for some restricted natural compounds; tamoxifen enhanced warfarin effects, and it is contraindicate that co-administration of tamoxifen and wafarin28,29). In our previous studies, we have been observed the possible interactions with Korean traditional polyherbal formulas; we observed that oral co-administration of Jaeumkanghwa-tang, a traditional yin-tonifying herbal medicine has been used for various oriental obstetrical and gynecological fields within 5 min did not critically influenced on the pharmacokinetics profiles of tamoxifen after single38) and repeated39) co-administration at dosage levels of 50mg/kg in tamoxifen and 100mg/kg in Jaeumkanghwa-tang, respectively.
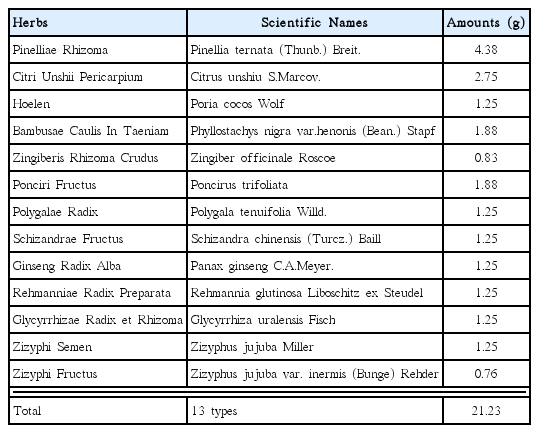
Gamiondam-tang (GMODT) consisted of 13 types of herbs - Pinelliae Rhizoma, Citri Unshii Pericarpium, Hoelen, Bambusae Caulis In Taeniam, Zingiberis Rhizoma Crudus, Ponciri Fructus, Polygalae Radix, Schizandrae Fructus, Ginseng Radix Alba, Rehmanniae Radix Preparata, Glycyrrhizae Radix et Rhizoma, Zizyphi Semen and Zizyphi Fructus and has been traditionally used to treat neuropsychiatric disorders such as neurosis and insomnia in traditional medicine 40,41). It has been reported that oral administration of GMODT improves cognitive function in aged rats through the increase of choline acetyltransferase expression in the basal forebrain42). Others also observed that GMODT prevents depressive behavior in thiamine-deficient mice and this may be closely related to the activation of cholinergic functions in the hippocampus43). Although some researchers have investigated the pharmacological effects of GMODT, there has been no study on its possible drug-drug interactions with tamoxifen.
In the present study, the effects of GMODT co-administration on the pharmacokinetics of tamoxifen were observed as a process of the comprehensive and integrative medicine, combination therapy of tamoxifen with GMODT to achieve synergic pharmacodynamics and reduce toxicity on the breast cancer patients.
Materials and methods
1. Animals and husbandry
A total of twenty-one male Sprague-Dawley (SD) rats (6-wk old upon receipt; OrientBio, Seungnam, Korea) were used after acclimatization for 12 days. Animals were allocated five per polycarbonate cage in a temperature (20–25°C) and humidity (40–45%) controlled room. Light : dark cycle was 12hr : 12hr and feed (Samyang, Korea) and water were supplied free to access. After twelve days of acclimatization, five rats per group were selected based on the body weights, and used further experiments in the present study. All animals were marked by picric acid, and overnight fasted (about 18hrs; water was not restricted) before treatment, and further fasted during 3hrs after end of treatment. Animal experiments were conducted according to the international regulations of the usage and welfare of laboratory animals, and approved by the Institutional Animal Care and Use Committee in Daegu Haany University (Gyeongsan, Gyeongbuk, Korea) [Approval No. DHU2013-059].
2. Test articles and formulation
Reddish brown granules of GMODT (HANZUNG PHARM. CO. LTD., Daejeon, Korea), produced according to Korean Good Manufacturing Practice and permitted and regulated by the Korean Food & Drug Administration (Seoul, Korea) were used in this experiment, and tamoxifen (Hangzhou Tacon Co., Ltd, Hangzhou, China) was used as control drug as listed follows. Individual compositions of ten kinds of herbs in GMODT were listed in Table 1. Tamoxifen and GMODT were stored in a refrigerator at 4°C to protect from light and degeneration until use. Both drugs are well dissolved (up to 20mg/ml solutions in GMODT and up to 10mg/ml solutions in tamoxifen) in distilled water as vehicle, respectively.
3. Groupings and administration
Five rats per group (two groups) were used in this study as follows. The doses of test materials were selected based on their toxicity and pharmacodynamics −50mg/kg of tamoxifen with 100mg/kg of GMODT. After 50mg/kg of tamoxifen treatment, GMODT 100 mg/kg was administered, within 5min. In tamoxifen single treated rats, 50mg/kg of tamoxifen was orally administered, and then distilled water 5ml/kg was orally administered, instead of GMODT solutions, 5 min-intervals. Each tamoxifen or GMODT was single orally administered, in a volume of 5ml/kg, dissolved in distilled water.
4. Plasma collections
All rats were anesthetized with 2 to 3% isoflurane (Hana Pharm. Co., Hwasung, Korea) in the mixture of 70% N2O and 28.5% O2, and blood samples (0.5 ml) were collected into 50 IU heparinized tubes via the orbital plexus at 30min before treatment (as a control), 30 min, 1, 2, 3, 4, 6, 8 and 24hrs after end of oral administration. Blood samples were immediately centrifuged for 10 min at 13,000 rpm and about 0.3 ml aliquots of plasma were stored in a −150°C deep freezer until analysis of tamoxifen.
5. Sample preparation and calibrations
Primary stock solution, 1.0mg/ml of tamoxifen in 100% MeOH (Baker, Phillipsburg, NJ, USA) and internal standard working solution, carbamazepine (Sigma-Aldrich, Sigma, St. Louise, MO, USA) 500 ng/ml in acetonitrile were prepared. Working standard solutions were prepared by dilution with acetonitrile. All standard solutions were stored at −20°C in the dark when not in use, and calibrated the standard samples as 100μl of blank plasma; working standard solutions and internal standard working solution were mixed with 200μl of acetonitrile. In addition, 100μl of sample plasma and internal standard working solution were mixed with 200μl of acetonitrile. The mixtures were mixed by vortex-mixing and centrifuged at 12,000 rpm for 10 min at 4°C. The clear supernatants (5.0μl) were transferred to injection vials and the aliquot was injected into the LC-MS/MS system.
6. LC-MS/MS conditions
Concentrations of tamoxifen in the rat plasma samples were determined LC-MS/MS method. Chromatographic analysis was performed using an Agilent 1100 Series HPLC (Agilent Technologies, Santa Clara, CA, USA) equipped with on-line degasser, binary pump, autosampler and column compartment. Separation of the analyte from potentially interfering material was achieved at ambient temperature using Waters SymmetryTM C18 columns (2.1×50mm, 3.5μm) (Waters Corp., Milford, MA, USA) at column oven 30°C. The mobile phase used for the chromatographic separation was composed of 50% distilled water (0.1% formic acid)/50% acetonitrile, and was delivered isocratically at a flow rate of 0.35ml/min. The column effluent was monitored using an API 2000 triple-quadruple mass-spectometric detector (Applied Biosystems, Foster City, CA, USA). The instrument was equipped with an electrospray interface in positive ion mode, and controlled by the Analyst version 1.4.1 software (Applied Biosystems, Foster City, CA, USA) (Linear (1/x2, no Iterate)). Samples were introduced to the interface through a Turbo IonSpray with the temperature set at 500°C. A high positive voltage of 4.0kV was applied to the ion spray. Nitrogen was used as the nebulizer gas, curtain gas, and collision gas with the settings of 70, 20, and 7, respectively. The multiple reaction monitoring (MRM) detection method was employed for the detection of tamoxifen; the transitions monitored were carbamazepine (IS): m/z 237>194 (Retention time: 0.63min), tamoxifen: 372>178 (Retention time: 0.55min). Calibration curves of tamoxifen were linear over the ranges studied with r2>0.999. The lower limit of quantification of the tamoxifen in the rat plasma was 8ng/ml.
7. Pharmacokinetic analysis
The plasma concentration data were analyzed using a noncompartmental method on commercial pharmacokinetics data analyzer programs (PK solutions 2.0; Summit, Montrose, CO, USA)44,45). The elimination rate constant (Kel) was calculated by the log-linear regression of tamoxifen concentration data during the elimination phase, and the terminal half-life (t1/2) was calculated by 0.693/Kel. The peak concentration (Cmax) and time to reach the peak concentration (Tmax) of tamoxifen in the plasma were obtained by visual inspection of the data in the concentration-time curve. The area under the plasma concentration-time curve (AUC0-t) from time zero to the time of the last measured concentration (Clast) was calculated using the linear trapezoidal rule46). The AUC zero to infinity (AUC0-inf) was obtained by adding AUC0-t and the extrapolated area was determined by Clast/Kel. The mean residence time infinity (MRTinf) was calculated by dividing the first moment of AUC (AUMC0-inf) by AUC0-inf.
8. Statistical analyses
All the means are presented with their standard deviation of five rats (Mean ± S.D. of five rat plasma concentrations of tamoxifen). The pharmacokinetic parameters were compared using a non-parametric comparison test, Mann-Whitney U (MW) test, on the SPSS for Windows (version 14.0K, SPSS Inc., USA). A p-value <0.05 was considered statistically significant. In addition, the percent changes between tamoxifen single formula treated rats and tamoxifen with GMODT co-administered rats were calculated to help the understanding of the effects of co-administration: Percentage changes as compared with tamoxifen 50mg/kg single treated mice (%) = [((Data of GMODT co-administrated rats – data of tamoxifen single formula treated rats)/Data of tamoxifen single formula treated rats) × 100].
Results
1. Changes on the plasma concentrations of tamoxifen
Tamoxifen was detected from 30 min to 24 hrs after end of administration in the both tamoxifen single or co-administered rats with GMODT, respectively. Noticeable increases trends of plasma concentration of tamoxifen were demonstrated until 1hr after end of co-administration, but then decreased throughout blood collecting points, especially significant (p<0.05) increases of the plasma tamoxifen concentrations were observed at 30min after co-administration of GMODT and tamoxifen as compared with tamoxifen single treated rats, at a dosage levels of 100mg/kg in GMODT and 50 mg/kg in tamoxifen (Fig 1). The plasma tamoxifen concentrations at 30 min, 1, 2, 3, 4, 6, 8 and 24hrs after end of treatment were changed as 69.30, 23.01, −5.86, −14.63, −26.95, −11.34, −12.47 and −48.66% in tamoxifen + GMODT treated rats as compared with tamoxifen single treated rats, respectively.
2. Changes on the Tmax of tamoxifen
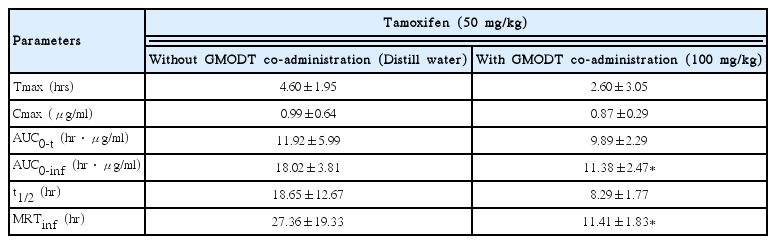
The Tmax of tamoxifen were non-significantly and slightly decreased as −43.48% in co-administrated rats with tamoxifen 50mg/kg and GMODT 100mg/kg (2.60±3.05hr) as compared with tamoxifen single treated rats (4.60±1.95hr), in the present study (Table 2).
3. Changes on the Cmax of tamoxifen
The Cmax of tamoxifen were non-significantly decreased as −12.34% in co-administrated rats with tamoxifen 50mg/kg and GMODT 100mg/kg (0.87±0.29 μg/ml) as compared with tamoxifen single treated rats 0.99±0.64μg/ml), in the present study (Table 2).
4. Changes on the AUC of tamoxifen
The AUC0-t of tamoxifen were non-significantly decreased as −17.02% in co-administrated rats with tamoxifen 50mg/kg and GMODT 100mg/kg (9.89±2.29hr · μg/ml) as compared with tamoxifen single treated rats (11.9216±5.99hr · μg/ml). In addition, AUC0-inf of tamoxifen were also significantly (p<0.05) decreased as −36.85% in co-administrated rats with tamoxifen and GMODT (11.38±2.47hr · μg/ml) as compared with tamoxifen single treated rats (18.02±3.81hr · μg/ml), in the present study (Table 2).
5. Changes on the t1/2 of tamoxifen
The t1/2 of tamoxifen were markedly but non -significantly decreased as −55.56% in co-administrated rats with tamoxifen 50mg/kg and GMODT 100 mg/kg (8.29±1.77hr) as compared with tamoxifen single treated rats (18.65±12.67hr), in the present study (Table 2).
6. Changes on the MRTinf of tamoxifen
The MRTinf of tamoxifen were markedly and significantly (p<0.05) decreased as −58.28% in co-administrated rats with tamoxifen 50 mg/kg and GMODT 100mg/kg (11.41±1.83hr) as compared with tamoxifen single treated rats (27.36±19.33hr), in the present study (Table 2).
Discussion
Co-administration with GMODT induced increased trends of plasma tamoxifen concentrations to 1hr after end of administration, and then showed decreased trends of plasma tamoxifen concentrations, and especially significant increases of plasma tamoxifen concentrations were demonstrated at 0.5hr after end of co-administration with GMODT and also related significant decreases of AUC0-inf and MRTinf as compared with tamoxifen single formula treated rats, at dosage levels of tamoxifen 10mg/kg and GMODT 100mg/kg within 5min, in this experiment. These findings are considered as direct evidences that GMODT significantly inhibited the oral bioavailability of tamoxifen through variable influences on the absorption and excretion of tamoxifen, can be influenced on the toxicity or pharmacodynamic of tamoxifen. Hence, it is recommended that pharmacokinetic studies should be tested like the effects of GMODT on the pharmacokinetics of tamoxifen, when they were co-administered with prolonger intervals than Tmax of tamoxifen oral administration (about 2.5 hr-intervals), to achieve the optimal dosing regimen of GMODT and tamoxifen co-administration, as a process of the comprehensive and integrative medicine, the combination therapy of tamoxifen with GMODT on the breast cancer.
Tamoxifen was absorbed slowly following oral administration and Tmax of tamoxifen occur about 3–6 hrs after a single dose47–49) but it rapidly and extensively metabolized in the liver, through a substrate of CYP3A, 2C9, 2D626 including an active major metabolite, N-desmethyltamoxifen has biologic activity similar to that of the parent drug50,51). Steady-state concentrations of tamoxifen are attained after 3–4 weeks and those of N-desmethyltamoxifen, an active metabolite, are attained after 3–8 weeks52). Tamoxifen excreted principally in feces as polar conjugates53) with about 5–7 days of t1/2 in tamoxifen and 9–14 days in N-desmethyltamoxifen48). Clearance of tamoxifen is higher in female children 2–10 years of age than in women54,55). In the present study, Tmax of tamoxifen in tamoxifen single oral treated rats was detected as 4.60±1.95hr, and Cmax, AUC0-t, AUC0-inf, t1/2 and MRTinf were detected as 0.99±0.64 μg, 11.92±5.99hr · μg/ml, 18.02±3.81hr · μg/ml, 18.65± 12.67hr and 27.36±19.33hr, respectively. In tamoxifen with GMODT co-administered rats, Tmax, Cmax, AUC0-t, AUC0-inf, t1/2 and MRTinf of tamoxifen were detected as 2.60±3.05hr, 0.87±0.29μg, 9.89±2.29hr · μg/ml, 11.38±2.47hr · μg/ml, 8.29±1.77hr and 11.41± 1.83hr as changed as −43.48, −12.34, −17.02, −36.85, −55.56 and −58.28% as compared with tamoxifen 50 mg/kg single oral treated rats, expecially significant decreases of AUC0-inf and MRTinf were observed in GMODT and tamoxifen co-administrated rats at dosage levels of tamoxifen 10mg/kg and GMODT 100mg/kg within 5min, as compared with tamoxifen single formula treated rats.
Tamoxifen rapidly and extensively metabolized in the liver, through a substrate of CYP3A, 2C9, 2D626 to active major metabolite, N-desmethyltamoxifen2,51) and, therefore, tamoxifen can be interacted with various drugs27–37). The severities of various side effects arise from tamoxifen treatment, especially bone loss56), endometrial cancer57), thromboembolism 58), fatty liver59), reduced cognition60), semantic memory scores61) and libido62,63), premature growth plate fusion64), immune suppression65,66) and hypersensitivity67,68) are considered as directly co-related with absorption and excretion of tamoxifen or pharmacodynamics. In the present study, single co-administration of GMODT with tamoxifen within 5 min significantly inhibited the oral bioavailability and retention time of tamoxifen through variable influences on the absorption and excretion of tamoxifen, can be influenced on the toxicity or pharmacodynamic of tamoxifen. Hence, it is considered that pharmacokinetic studies should be tested like the effects of GMODT on the pharmacokinetics of tamoxifen, when they were co-administered with prolonger intervals than Tmax of tamoxifen oral administration, about 2.5 hr-intervals, to achieve the optimal dosing regimen of GMODT and tamoxifen co-administration, as a process of the comprehensive and integrative medicine, the combination therapy of tamoxifen with GMODT on the breast cancer.
Conclusions
Based on the results of the this study, it is considered that single co-administration GMODT within 5min significantly inhibited the oral bioavailability of tamoxifen through variable influences on the absorption and excretion of tamoxifen, can be influenced on the toxicity or pharmacodynamic of tamoxifen. Therefore, it is recommended that pharmacokinetic studies should be tested like the effects of GMODT on the pharmacokinetics of tamoxifen, when they were co-administered with prolonger intervals than Tmax of tamoxifen oral administration (about 2.5 hr-intervals), to achieve the optimal dosing regimen of GMODT and tamoxifen co-administration, as a process of the comprehensive and integrative medicine, the combination therapy of tamoxifen with GMODT on the breast cancer.
Acknowledgments
This study was supported by grant of Korea of Health & Welfare, Republic of Korea (Project No: 20-11-0-090-091-3000-3033-320).