References
1. 2016 Statistical Indicator of Medical Expenses Health Insurance Review & Assessment service; 2017. p. 1–16.
2. Mudarri DH. Valuing the Economic Costs of Allergic Rhinitis, Acute Bronchitis, and Asthma from Exposure to Indoor Dampness and Mold in the US. Journal of Environmental and Public Health 2016;2016:2386596.
3. Eom SJ. Essentials of Primary Care: Respiratory: The most common disease of Koreans, Acute bronchitis. The Korean Association of International Medicine Sping Conference Collection 2016;2016:122–5.
4. Albert RH. Diagnosis and treatment of acute bronchitis. Am Fam Physician 2010;82(11):1345–50.
5. Pratter MR. Cough and the common cold: ACCP evidence-based clinical practice guidelines. Chest 2006;129:72S–4S.
6. Wenzel RP, Fowler AA 3rd. Clinical practice. Acute bronchitis. N Engl J Med 2006;355(20):2125–2130.
7. Cough Guideline The Korean Academy of Tuberculosis and Respiratory Diseases; 2014. p. 26–34.
8. Dempsey PP, Businger AC, Whaley LE, Gagne JJ, Linder JA. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC family practice 2014;15(1):194.
9. Smith SM, Fahey T, Smucny J, Becker LA. Antibiotics for acute bronchitis. Cochrane Database Syst Rev 2014;3:CD000245.
10. Gonzales R, Bartlett JG, Besser RE, Cooper RJ, Hickner JM, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Annals of Internal Medicine 2001;134(6):521–529.
11. Tackett KL, Atkins A. Evidence-based acute bronchitis therapy. Journal of pharmacy practice 2012;0897190012460826.
12. Braman SS. Chronic cough due to acute bronchitis: ACCP evidence-based clinical practice guidelines. Chest 2006;129(suppl 1):95S–103S.
13. Knutson D, Braun C. Diagnosis and management of acute bronchitis. Am Fam Physician 2002;65(10):2039–2044.
14. Becker LA, Hom J, Villasis-Keever M, van der Wouden JC. Beta2-agonists for acute cough or a clinical diagnosis of acute bronchitis. Cochrane Database Syst Rev 2015;
15. Jiang L, Li K, Wu T. Chinese medicinal herbs for acute bronchitis. The Cochrane Library 2012;
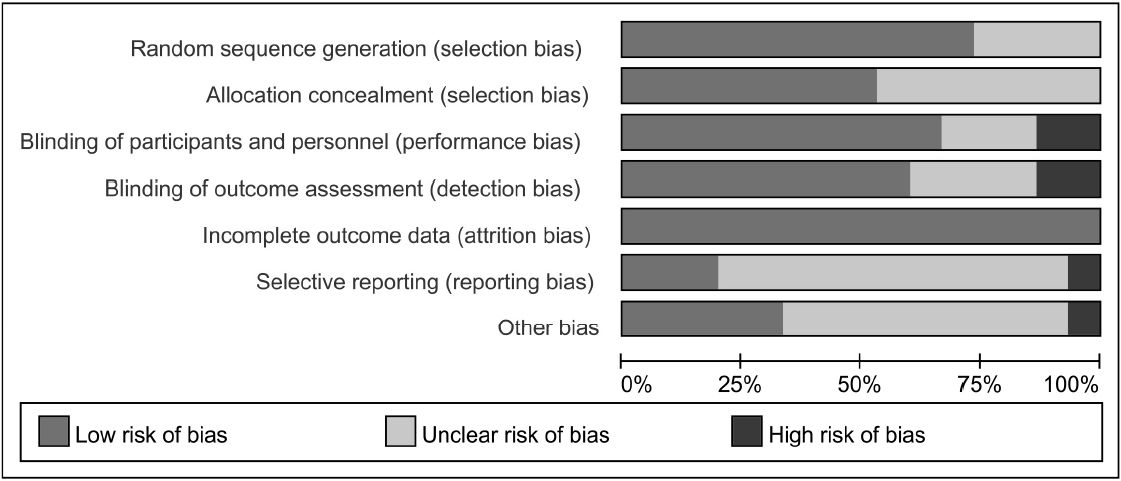
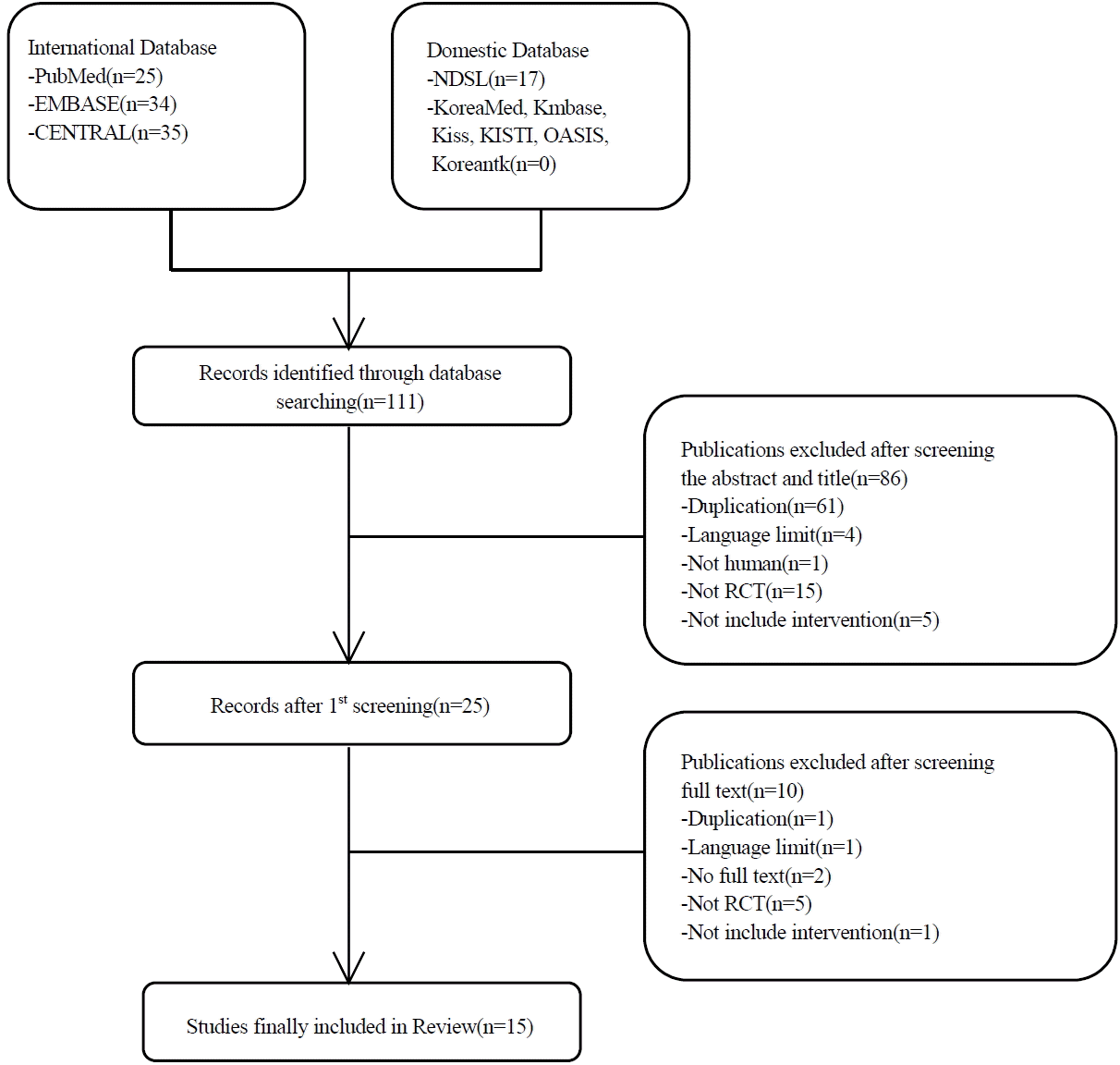
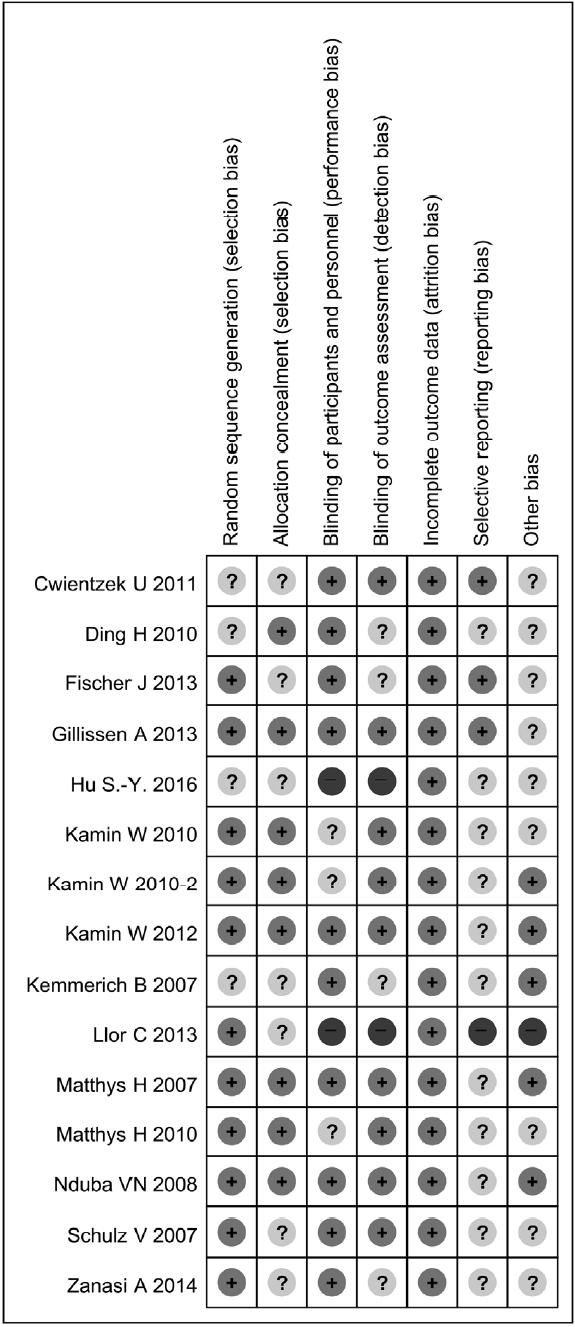
16. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Sterne JA, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 3432011;:d5928.
17. Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011;
18. Kinkade S, Natalie AL. Acute Bronchitis. American Family Physician 94:7. 2016;
19. Lehrl S, Matthys H, Kamin W, Kardos P. The BSS—a valid clinical instrument to measure the severity of acute bronchitis. Pulm Respir Res 12014;:00016.
20. Matthys H, Kamin W. Positioning of the Bronchitis Severity Score (BSS) for standardised use in clinical studies. Current medical research and opinion 29(10)2013;:1383–1390.
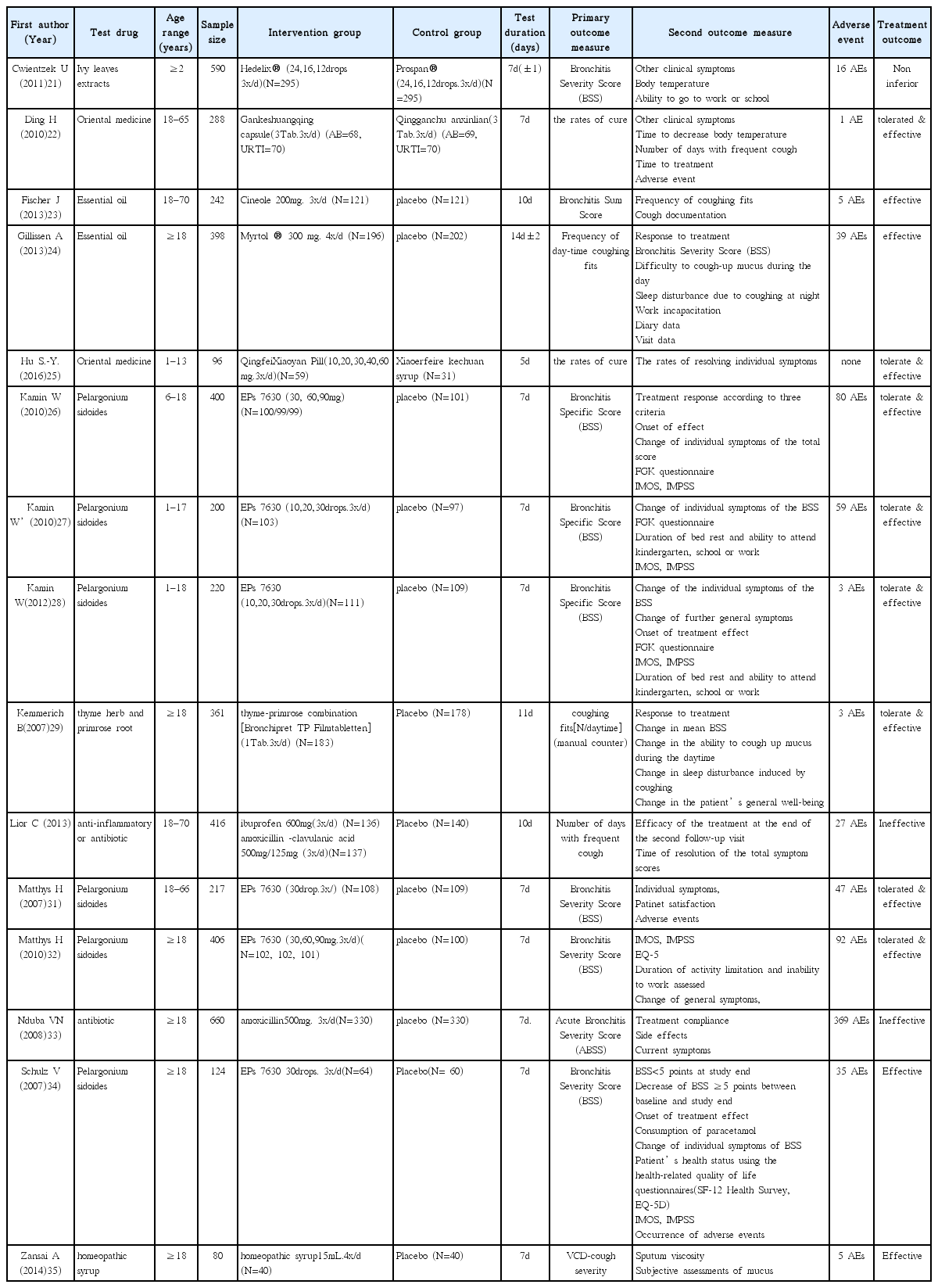
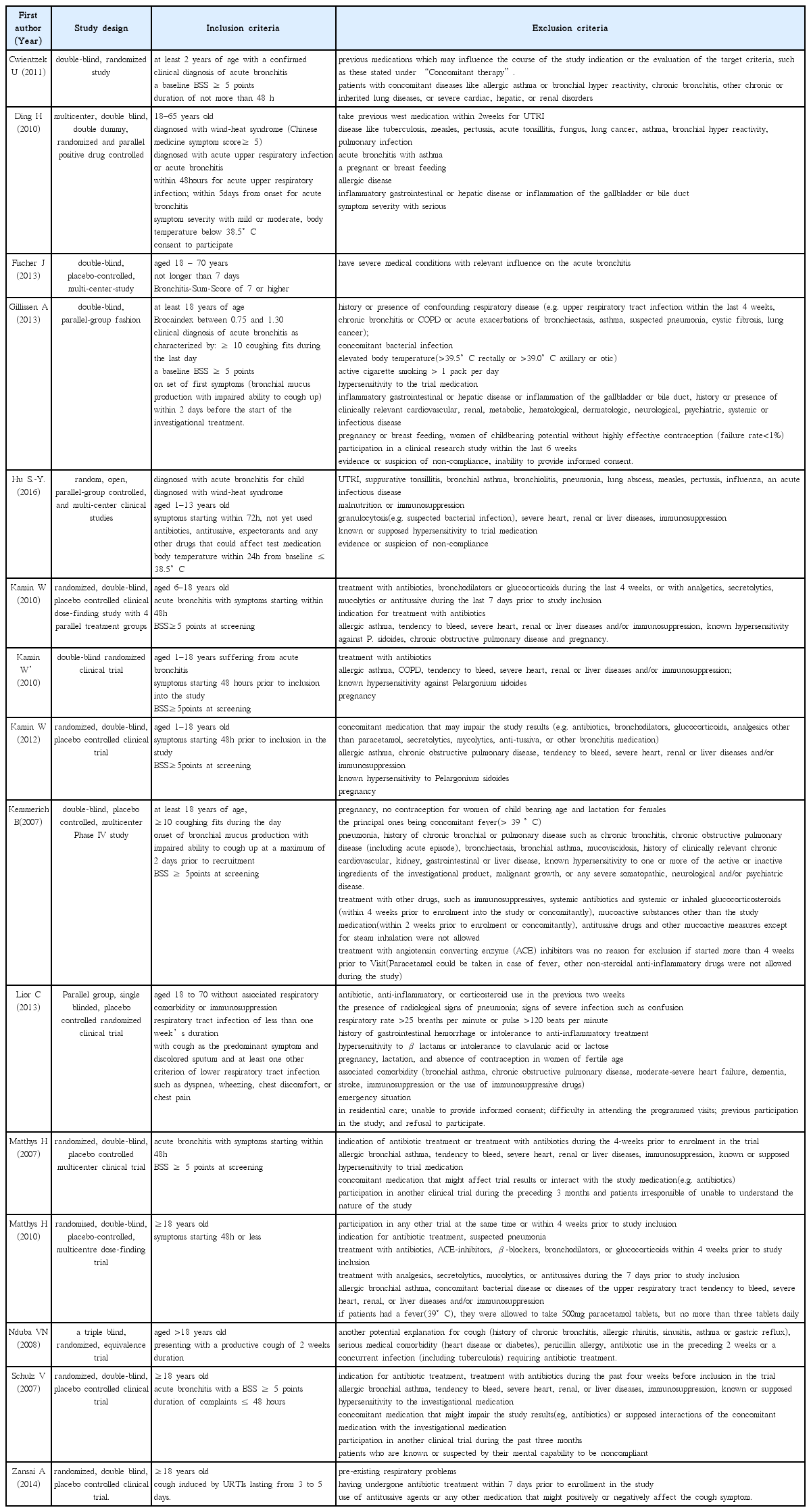
21. Cwientzek U, Bertram O, Petr A. Acute bronchitis therapy with ivy leaves extracts in a two-arm study. A double-blind, randomised study vs. an other ivy leaves extract. Phytomedicine 18(13)2011;:1105–1109.
22. Ding H, Yang Mj, Lv B, Dong Y, Li Xj, Luo Wj, et al. A Multicentered, Double-blind, Randomized Controlled Trials of Gankeshuangqing Capsule in the Treatment of Wind-heat Syndrome (Acute Upper Respiratory Infection or Acute Bronchitis). Chinese journal of evidence-based medicine 2010;10(1):14–22.
23. Fischer J, Dethlefsen U. Efficacy of cineole in patients suffering from acute bronchitis: a placebo-controlled double-blind trial. Cough 2013;9(1):25.
24. Gillissen A, Wittig T, Ehmen M, Krezdorn HG, de Mey C. A multi-centre, randomised, double-blind, placebo-controlled clinical trial on the efficacy and tolerability of GeloMyrtol® forte in acute bronchitis. Drug research 63(01)2013;:19–27.
25. Hu SY, Li JH, Tang F, Cheng Y, Zhang YM, Zhang PP, et al. Clinical observation of Qingfei Xiaoyan Pill in treatment of lung phlegm heat syndrome in children with acute bronchitis. [Chinese]. Chinese Traditional and Herbal Drugs 2016;47(10):1746–9.
26. Kamin W, Maydannik VG, Malek FA, Kieser M. Efficacy and tolerability of EPs 7630 in patients (aged 6–18 years old) with acute bronchitis. Acta Paediatrica 99(4)2010;:537–543.
27. Kamin W, Maydannik V, Malek FA, Kieser M. Efficacy and tolerability of EPs 7630 in children and adolescents with acute bronchitis-a randomized, double-blind, placebo-controlled multicenter trial with a herbal drug preparation from Pelargonium sidoides roots. International journal of clinical pharmacology and therapeutics 48(3)2010;:184–191.
28. Kamin W, Ilyenko LI, Malek FA, Kieser M. Treatment of acute bronchitis with EPs 7630: randomized, controlled trial in children and adolescents. Pediatrics International 54(2)2012;:219–226.
29. Kemmerich B. Evaluation of Efficacy and Tolerability of a Fixed Combination of Dry Extracts of Thyme Herb and Primrose Root in Adults Suffering from Acute Bronchitis with Productive Cough. Arzneimittelforschung 57(09)2007;:607–615.
30. Llor C, Moragas A, Bayona C, Morros R, Pera H, Plana-Ripoll O, Miravitlles M, et al. Efficacy of anti-inflammatory or antibiotic treatment in patients with non-complicated acute bronchitis and discoloured sputum: randomised placebo controlled trial. bmj 3472013;:f5762.
31. Matthys H, Heger M. Treatment of acute bronchitis with a liquid herbal drug preparation from Pelargonium sidoides (EPs 7630): a randomised, double-blind, placebo-controlled, multicentre study. Current medical research and opinion 23(2)2007;:323–331.
32. Matthys H, Lizogub VG, Malek FA, Kieser M. Efficacy and tolerability of EPs 7630 tablets in patients with acute bronchitis: a randomised, double-blind, placebo -controlled dose-finding study with a herbal drug preparation from Pelargonium si doides. Current medical research and opinion 26(6)2010;:1413–1422.
33. Nduba VN, Mwachari CW, Magaret AS, Park DR, Kigo A, Hooton TM, Cohen CR. Placebo found equivalent to amoxicillin for treatment of acute bronchitis in Nairobi, Kenya: a triple blind, randomised, equivalence trial. Thorax 63(11)2008;:999–1005.
34. Schulz V. Liquid herbal drug preparation from the root of Pelargonium sidoides is effective against acute bronchitis: results of a double-blind study with 124 patients. Phytomedicine 142007;:74–75.
35. Zanasi A, Mazzolini M, Tursi F, Morselli-Labate AM, Paccapelo A, Lecchi M. Homeopathic medicine for acute cough in upper respiratory tract infections and acute bronchitis: A randomized, double-blind, placebo-controlled trial. Pulmonary pharmacology & therapeutics 27(1)2014;:102–108.
36. Traditional Korean Medicine Clinical Practice Guideline -Antitussive and Protussive Therapy Korea Food & Drug Administration; 2006. p. 1–21.
37. Kim KI, Lee HJ, Lee BJ, Jung HJ, Jung SK, Lee JH. Analysis of Recent Clinical Studies to Establish Korean Herbal Medicine Clinical Trial Guidelines for the Common Cold. J Int Korean Med 2016;37(1):109–134.
38. The Korean Academy of Tuberculosis and Respiratory Diseases. Respiratory Diseases 3rd rev edth ed. Soeul: Koonja publishing INC; 2007. p. 695–703.
39. Kardos P, Lehrl S, Kamin W, Matthys H. Assessment of the effect of pharmacotherapy in common cold/acute bronchitis–the Bronchitis Severity Scale (BSS). Pneumologie 68(08)2014;:542–546.
40. Leconte S, Ferrant D, Dory V, Degryse J. Validated methods of cough assessment: a systematic review of the literature. Respiration 81(2)2010;:161–174.
41. Schmit KM, Coeytaux RR, Goode AP, McCrory DC, Yancy WS, Kemper AR, Sanders GD, et al. Evaluating cough assessment tools: a systematic review. CHEST Journal 144(6)2013;:1819–1826.
42. Yousaf N, Lee KK, Jayaraman B, Pavord ID, Birring SS. The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute). Cough 7(1)2011;:4.
43. Han JM, Jung IC, Kang W, Kim SS, Yeo Y, Park YC. Reliability and validity of leicester cough questionnaire Korean version. Chronic respiratory disease 11(3)2014;:147–152.

 TP Filmtabletten] (1Tab.3x/d) (N=183)
TP Filmtabletten] (1Tab.3x/d) (N=183)