Strategic Development Plan of Standardization on Korean Medicine: Comparison with Traditional Medicine Policies between Korea and China
Article information
Abstract
Objectives
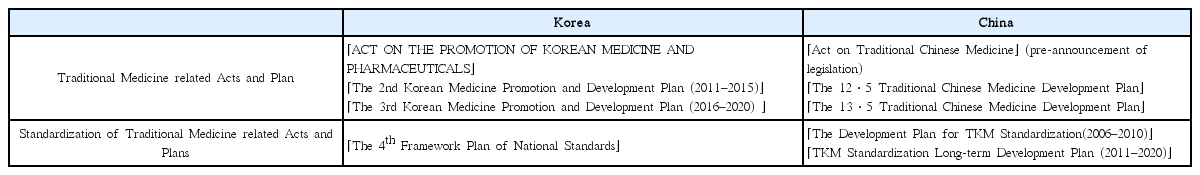
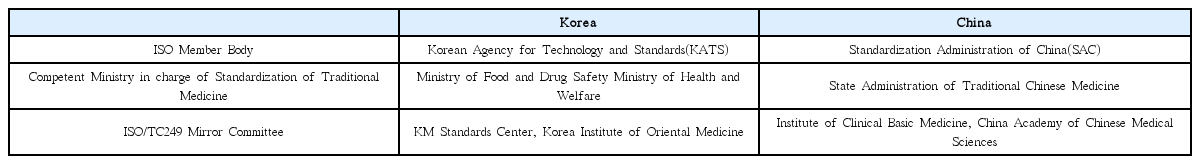
This study aims to investigate the policy status of traditional medicine standardization between Korea and China as well as to derive a strategic development plan for Korean medicine standardization.
Methods
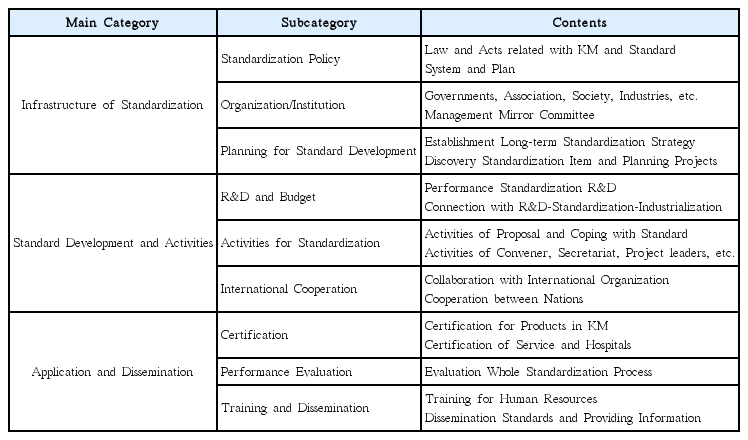
The existing national plans and preceding studies on traditional medicine standardization in Korea and China were reviewed, and scope of the study was categorized as having three main aspects: infrastructure; standard development and standardization activities; and application and diffusion.
Results
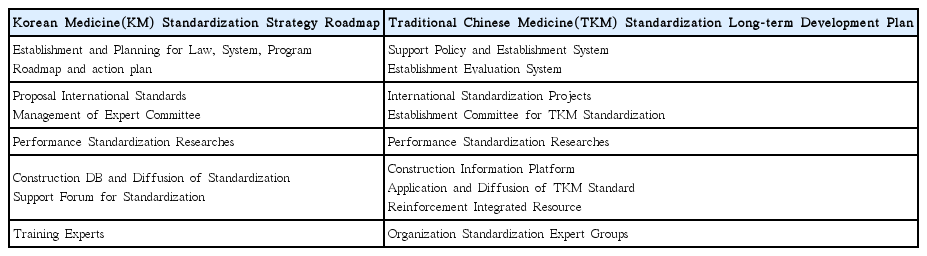
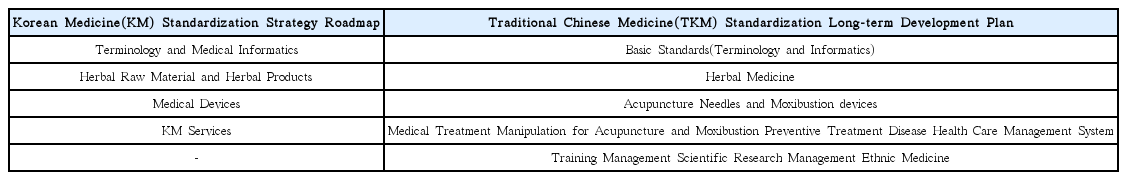
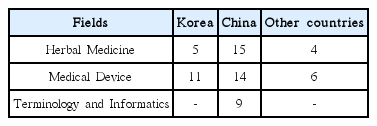
Nine development plans for Korean medicine standardization derived under the study were as follows: With regard to infrastructure of standardization, 1) standardization plan of Korean medicine shall be established based on involvement of multi-government ministries. 2) It shall be set with a consistent cooperation process among standardization-related organizations, as well as 3) encourage the industry to participate in standardization activities. To activate standards development, 4) launching the R&D-standardization-industrialization linked project, 5) supporting Korean medicine society and the mirror committee for motivation, and 6) planning the international joint research for development of standard and expanding standardization activities are recommended. In the aspect of application, there is a need for 7) expanding certification systems for the industry and 8) evaluation and feedback on the life-cycle of standardization. Lastly, 9) educational programs for training experts of standardization shall be developed and implemented.
Conclusions
In order to invigorate standardization activities for Korean medicine, enhancement of planning and evaluation capacity, ensuring the strategic development of standards, broadening boundaries of international standardization activities, and training professionals are required.
References
ISO/IEC Directives Part1. 2016