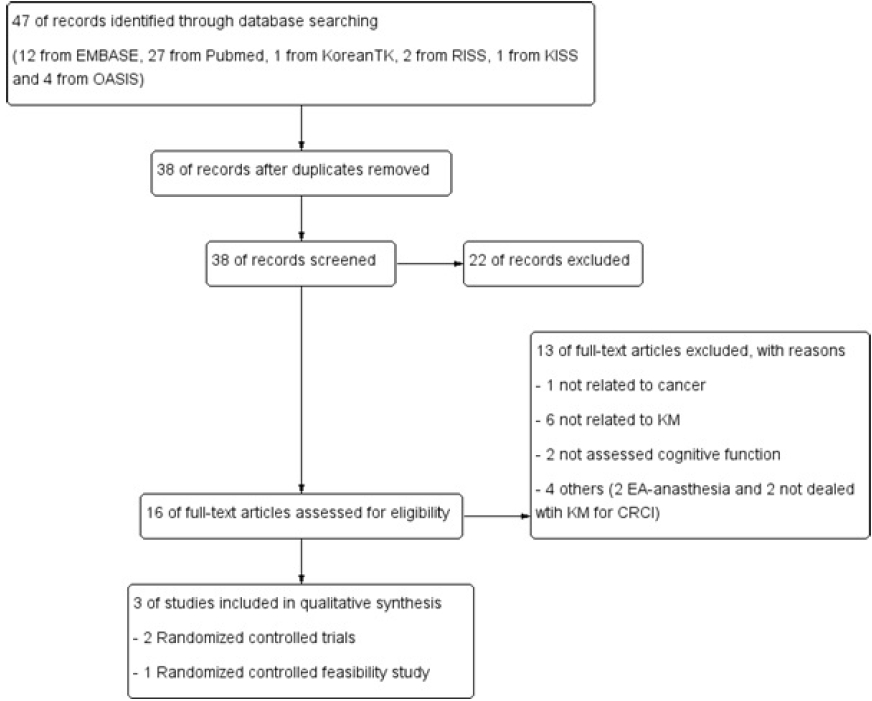
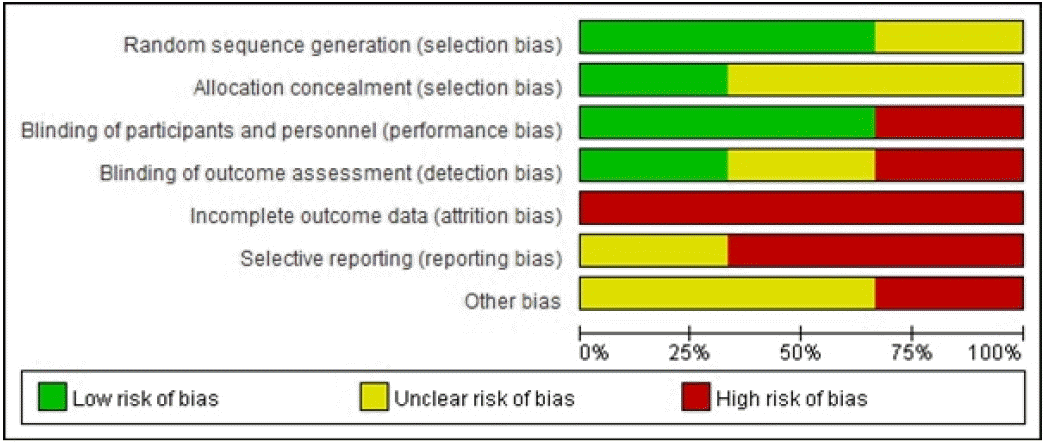
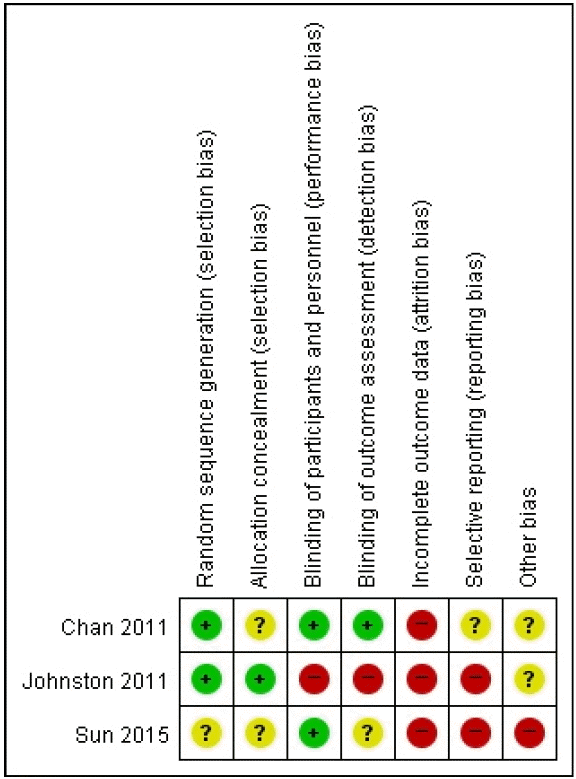
References
1. Denlinger CS, Ligibel JA, Are M, Baker KS, Demark-Wahnefried W, Friedman DL, et al. Survivorship: cognitive function, version 1.2014. Journal of the National Comprehensive Cancer Network : JNCCN 2014;12(7):976–86.
2. Cheung YT, Chui WK, Chan A. Neurocognitive impairment in breast cancer patients: pharmacological considerations. Critical reviews in oncology/hematology 2012;83(1):99–111.
3. Park JH, Bae SH, Jung YS, Jung YM. Prevalence and Characteristics of Chemotherapy-related Cognitive Impairment in Patients with Breast Cancer. J Korean Acad Nurs 2015;45(1):118–28.
4. Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34(6):611–35.
5. Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. The Lancet Oncology 2011;12(7):703–8.
6. Kohli S, Griggs JJ, Roscoe JA, Jean-Pierre P, Bole C, Mustian KM, et al. Self-reported cognitive impairment in patients with cancer. Journal of oncology practice/American Society of Clinical Oncology 2007;3(2):54–9.
7. Ahles TA, Correa DD. Neuropsychological impact of cancer and cancer treatments. Psycho-oncology 2010;:251–7.
8. Palmer JL, Trotter T, Joy AA, Carlson LE. Cognitive effects of Tamoxifen in pre-menopausal women with breast cancer compared to healthy controls. Journal of cancer survivorship : research and practice 2008;2(4):275–82.
9. Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28(8):1294–300.
10. Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast cancer research and treatment 2008;110(1):143–52.
11. Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2011;19(10):1647–56.
12. Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. International review of psychiatry (Abingdon, England) 2014;26(1):102–13.
13. Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Current neurology and neuroscience reports 2012;12(3):267–75.
14. Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. Journal of Clinical Oncology 2007;25(17):2455–63.
15. Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer journal (Sudbury, Mass) 2008;14(6):396–400.
16. Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. Journal of Clinical Oncology 2012;30(10):1080–6.
17. de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Human brain mapping 2012;33(12):2971–83.
18. Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Human brain mapping 2011;32(3):480–93.
19. Kesler SR, Watson C, Koovakkattu D, Lee C, O’Hara R, Mahaffey ML, et al. Elevated prefrontal myo-inositol and choline following breast cancer chemotherapy. Brain imaging and behavior 2013;7(4):501–10.
20. Janelsins MC, Mustian KM, Palesh OG, Mohile SG, Peppone LJ, Sprod LK, et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2012;20(4):831–9.
21. Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature reviews. Cancer 2007;7(3):192–201.
22. Raffa RB. A proposed mechanism for chemotherapy-related cognitive impairment (‘chemo-fog’). Journal of clinical pharmacy and therapeutics 2011;36(3):257–9.
23. Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA: a cancer journal for clinicians 2015;65(2):123–38.
24. Ju C. Hwang Jae Nae Kyeong Yeongchu Cheonghong; 2007.
25. Committee for the textbook of Traditional Korean neuropsychiatry. Traditonal Korean medical Neuropsychiatry 1Jipmoon; 2007.
26. Committee for the textbook of the acupuncture and moxibustion. Amnesia and Dementia. The acupuncture and moxibustion 3 2Jipmoon; 2008.
27. Lee BH, Kim HY, Park JH, Yang TY, Jang EY, Jeon HS, et al. Effect of acupuncture on the short term memory. The journal of East-West medicine 2015;40(3):13–23.
29. Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. Journal of cancer survivorship : research and practice 2009;3(4):223–32.
30. Towler P, Molassiotis A, Brearley S. What is the evidence for the use of acupuncture as an intervention for symptom management in cancer supportive and palliative care: an integrative overview of reviews. Supportive Care in Cancer 2013;21(10):2913–23.
31. Liu F, Li ZM, Jiang YJ, Chen LD. A meta-analysis of acupuncture use in the treatment of cognitive impairment after stroke. Journal of alternative and complementary medicine (New York, NY) 2014;20(7):535–44.
32. Cao H, Wang Y, Chang D, Zhou L, Liu J. Acupuncture for vascular mild cognitive impairment: a systematic review of randomised controlled trials. Acupuncture in medicine : journal of the British Medical Acupuncture Society 2013;31(4):368–74.
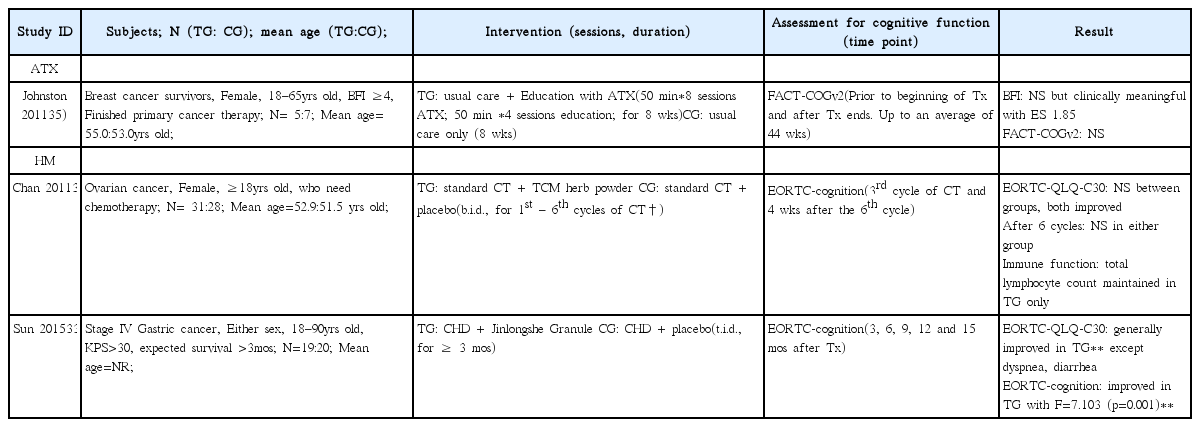
33. Sun DZJ, Zhang JP, Xu X, Ye JY, Xiu M, Zhao LJ, et al. Therapeutic effect of Jinlongshe Granule () on quality of life of stage IV gastric cancer patients using EORTC QLQ-C30: A double-blind placebo-controlled clinical trial. Chinese journal of integrative medicine 2015;21(8):579–86.
34. Chan KKY, Jones TJ, Zhao B, Ma JF, Leung FK, Lau CY, et al. The use of Chinese herbal medicine to improve quality of life in women undergoing chemotherapy for ovarian cancer: a double-blind placebo-controlled randomized trial with immunological monitoring. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2011;22(10):2241–9.
35. Johnston MFH, Subramanian RD, Elashoff SK, Axe RM, Li EK, Kim JJ, et al. Patient education integrated with acupuncture for relief of cancer-related fatigue randomized controlled feasibility study. BMC complementary and alternative medicine 2011;11:49.
36. Yun YH, Park YS, Lee ES, Bang SM, Heo D, Park S, et al. Validation of the Korean version of the EORTC QLQ-C30. Quality of Life Research 2004;13(4):863–8.
37. Deng M, Wang XF. Acupuncture for amnestic mild cognitive impairment: a meta-analysis of randomised controlled trials. Acupuncture in Medicine 2016;acupmed-2015-010989.
38. Hwang D. Bang-Yak-Hap-Pyun(方藥合編). Younglim 2010;
39. LI Cj, WEI Pk, YU Bl. Study on the mechanism of Xiaotan Sanjie Recipe for inhibiting proliferation of gastric cancer cells. Journal of Traditional Chinese Medicine 2010;30(4):249–53.
40. Joo SK, Kim GW, Goo BS, Kim CH. Effects of Arisaematics rhizoma on Ischemic Damage and Cytotoxicity in Brain. Journal of Korean Medicine 2001;2(1)
41. Won HY, Choi CW, Kim KS, Kim KO, Lee DW, Kim SY. The effects of Banhabaekchulchunma-Tang (BCT) on Dementia induced by focal brain ischemic injury in rats. Journal of Oriental Neuropsychiatry 2006;17(2):61–73.
42. Kim S, Ryu B, Ryu K, Park D. Study of (386) 대한한의학회지 제 37 권 제 3 호 (2016 년 9 월) anti-cancer effects of Cremastrae Appendiculatae Tuber on stomach cancer cells. J Korean Oriental Med 2001;22(2):75–83.
43. Seo B, Lee E. A Philological Study on poisoning of Pseudobulbus Cremastrae Appendiculatae. Journal of applied oriental medicine 2003;3(1):73–82.
44. Kim H, Hong S. The anti-inflammatory effects of Huang-Lyun (Coptidis Rhizoma, CR) on injured tissue after burn elicitation. J Korean Oriental Med 2011;32(2):1–13.
45. Jeong MY, Kim YH, Lee NK, Lee JY, Herr Y, Lee JH, et al. Antimicrobial effect on the periodontal pathogens and anti-inflammatory effect of Eriobotryae Folium. J Korean Orient Med 2008;29:182–92.
46. Avisar AR, Schiff Y, Bar-Sela E, Steiner G, Ben-Arye M. Chemotherapy-related cognitive impairment: does integrating complementary medicine have something to add? Review of the literature. Breast cancer research and treatment 2012;136(1):1–7.
47. Johnston MFY, Hui C, Xiao KK, Li B, Rusiewicz X. Acupuncture for chemotherapy -associated cognitive dysfunction: a hypothesis -generating literature review to inform clinical advice. Integrative cancer therapies 2007;6(1):36–41.
48. Yu SJ, Tseng J, Fu Ling. a Chinese herbal drug, modulates cytokine secretion by human peripheral blood monocytes. International journal of immunopharmacology 1996;18(1):37–44.
49. Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. The Journal of neuroscience 2006;26(42):10709–16.
50. O’Sullivan E, Higginson I. Clinical effectiveness and safety of acupuncture in the treatment of irradiation-induced xerostomia in patients with head and neck cancer: a systematic review. Acupuncture in Medicine 2010;28(4):191–9.
51. Sun X, Zhang X, Nian JY, Guo J, Yin Y, Zhang GL, et al. Chinese Herbal Medicine as Adjunctive Therapy to Chemotherapy for Breast Cancer: A Systematic Review and Meta -Analysis. Evidence-based complementary and alternative medicine : eCAM 2016;2016:3281968.