Evaluation of the effects of a low dose of Asiasari radix on stem cell morphology and proliferation
Article information
Abstract
Objectives
Asiasari radix (A. radix) is a traditional herb medicine that has been used as an analgesic, antitussive, or anti-allergic remedy. This study was performed to evaluate the effects of low concentration of the Asiasarum heterotropoides extract on the morphology and proliferation of the human mesenchymal stem cells derived from periodontal tissue.
Methods
Stem cells derived from gingiva were grown in the presence of A. radix at final concentrations that ranged from 0.001 to 0.01 μg/mL. The morphology of the cells was viewed under an inverted microscope and the analysis of cell proliferation was performed by using Cell Counting Kit-8 (CCK-8) on days 1 and 3.
Results
The control group showed fibroblast morphology. The shapes of the cells in 0.001 and 0.01 μg/mL of A. radix were similar to that of the untreated control group. The cultures growing in the presence of A. radix at day 1 showed an increase in the CCK-8 value. The relative values of CCK-8 assays of 0.001 and 0.01 μg/mL of A. radix are 130.6 % ± 1.8 % and 129.3 % ± 1.5 %, respectively, when the CCK-8 result of the untreated control group at day 1 is considered 100% (P = 0.051).
Conclusions
Within the limits of this study, low concentrations of A. radix seemed to increase the proliferation of the stem cells that were derived from the gingiva and did not have adverse effects on the morphology of the cells.
Introduction
Asiasari Radix (A. radix) is a traditional herb that is widely used to treat various diseases in Korea and China1). A. radix is primarily derived from Asiasarum heterotropoides or Asiasarum sieboldii2–3). It has been used as an analgesic, antitussive, or anti-allergic remedy for generations4). It has also been used to treat dental diseases that include aphthous stomatitis, toothache, and gingivitis1,5). In Dongeubogam, there are many prescriptions of main blended A. radix, and those were the most frequently used to treat dental diseases that incuded gingivitis6). However, only limited information is currently available regarding the effects of A. radix on dental tissue, including mesenchymal stem cells derived from gingiva.
Several studies were performed to evaluate the effects of A. radix on cell proliferations with conflicting results3–5,7,8). A. radix had no significant effects on the growth of HeLa cells at a range of concentrations (0.0001 to 1,000 μg/mL) at 72 hr7). Similarly, neither the proliferation nor the viability of whole spleen cells or human U266B1 multiple myeloma cells were affected with 1000 μg/mL of A. radix4). However, in a study on a human colorectal carcinoma cell line (HCT-116 cells), increasing concentrations (0–50 μg/mL) of ethanol extract of A. radix for 48 h were shown to repress the cell proliferation in a dose-dependent manner8). Similarly, our previous study showed that the groups treated with 100 and 1,000 μg/mL (tested range of concentrations of 0.1 to 1,000 μg/mL) repressed the proliferation 3). On the other hand, A. radix at a dose of 0.1 μg/mL increased the proliferation of an immortalized human keratinocyte cell line and the primary cultures of human hair dermal papilla cells5).
Limited information is currently available regarding the effects of A. radix on the oral tissue3). Our groups also evaluated the effects of other traditional herb including Angelicae dahuricae radix and Cimicifugae Rhizoma on mesenchymal stem cells derived from ginigiva9,10). This study was performed to evaluate the effects of the extract of Asiasarum heterotropoides at a low concentration on the morphology and proliferation of the human mesenchymal stem cells derived from periodontal tissue.
Methodology
1. Preparation of the materials
The dry roots of Asiasarum heterotropoides (400 g) were immersed in distilled water and boiled under reflux for 2 h 30 min. The resulting extract was centrifuged and the supernatant was concentrated to 300 mL by using a rotary evaporator (Eyela NE-1001, Tokya Rikakikai Co. Ltd, Tokyo, Japan) under reduced pressure. The concentrates were then freeze-dried in lyophilizer (Labconco, Kansas, USA). Sixty-five gram of solid residue was obtained and the yield was 16% (w/w).
2. Isolation and culture of the stem cells derived from the gingiva
Healthy gingival tissue samples were obtained from healthy patient during crown lengthening procedures. Crown lengthening procedures are performed to provide adequate room for crown preparation and reestablishment of the biologic width between the crown margin and the bone crest11). This study was reviewed and approved by the Institutional Review Board of Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea (KC11SISI0348), and informed consents were obtained from the patients.
The resected gingival tissues were immediately placed in sterile phosphate-buffered saline (PBS, Welgene, Daegu, Korea) containing penicillin, and streptomycin (Sigma-Aldrich Co., St. Louis, USA) at 4°C. The tissue was de-epithelialized, and digested with collagenase IV (Sigma-Aldrich Co.). Then the cells were incubated at 37°C in a humidified incubator. The non-adherent cells were washed with PBS (Welgene), replaced with fresh medium, and fed every 2–3 days.
3. Evaluation of stem cell morphology
The stem cells were plated at a density of 2.0 ×103 cells/well in 96-well plates. The cells were incubated in Minimum Essential Medium α (α-MEM, Gibco, Grand Island, USA) with 10 mM ascorbic acid 2-phosphate (Sigma-Aldrich Co.), 200 mM L-Glutamine (Sigma-Aldrich Co.), 15% fetal bovine serum (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich Co.) in the presence of the A. radix at final concentrations that ranged from 0 to 0.01 μg/mL (0 (untreated control), 0.001 and 0.01 μg/mL), respectively. The morphology of the cells was viewed under an inverted microscope (Leica DM IRM, Leica Microsystems, Wetzlar, Germany) on days 1 and 3.
4. Determination of cell proliferation
The analysis of cell proliferation was performed on days 1 and 3. Cell Counting Kit-8 (CCK-8, Dojindo, Tokyo, Japan) assay was used to identify viable cells. The spectrophotometric absorbance was measured at 450 nm with a microplate reader (BioTek, Winooski, USA). The experiments were done in triplicate.
5. Statistical Analysis
The findings are represented as the means ± standard deviations of the experiments. A test of normality was performed and Levene’s test of homogeneity of variances revealed the P-value of 0.040. Non-parametric statistical procedures with Kruskal-Wallis methods were used to test for differences between three groups with commercially available statistical software (SPSS 12 for Windows, SPSS Inc., Chicago, IL, USA).
Results
1. Evaluation of cell morphology
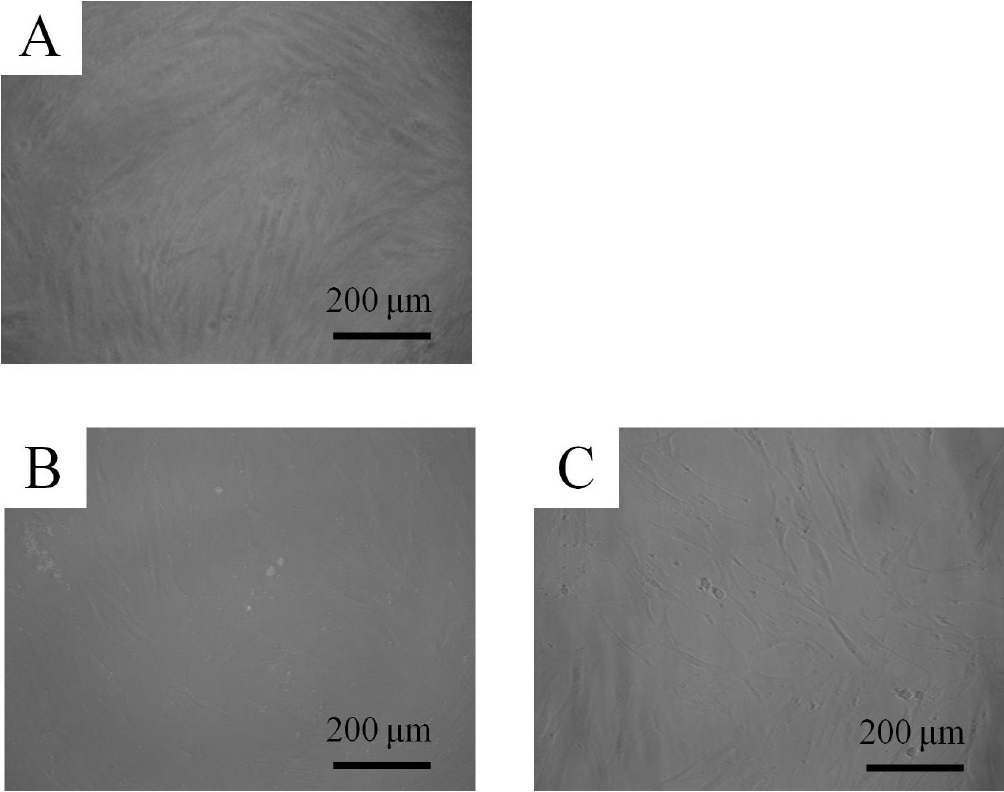
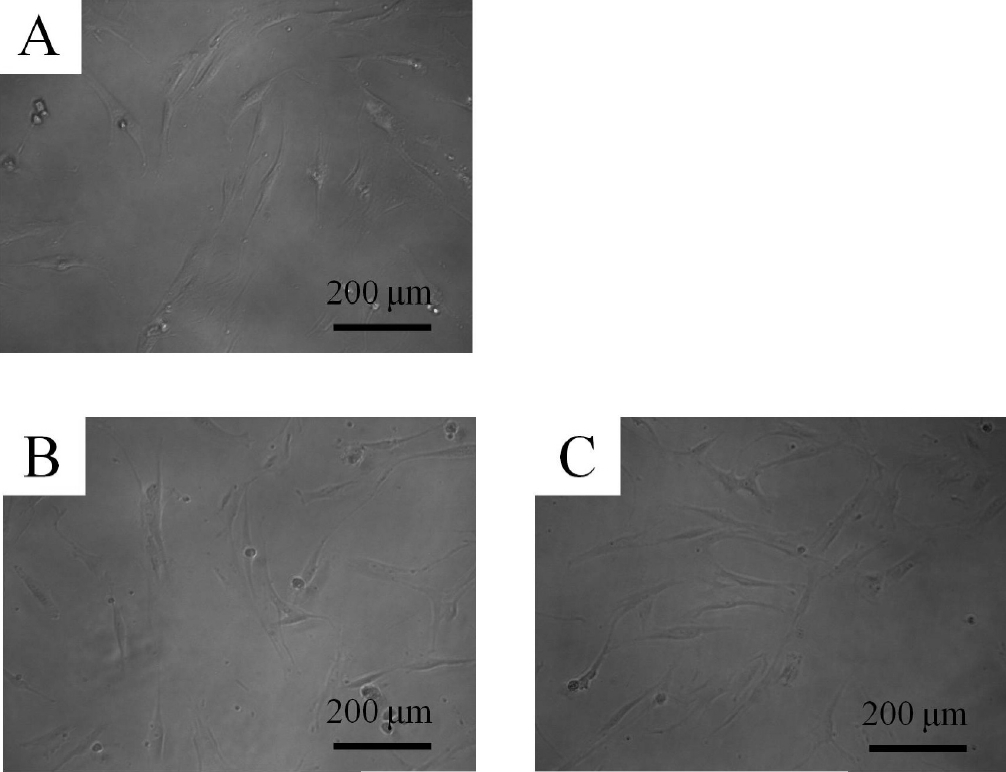
These cells showed colony-forming abilities, plastic adherence, and multilineage differentiation (osteogenic, adipogenic, chondrogenic) potency and expressed CD44, CD73, CD90, and CD105, but did not express CD14, CD45, CD34, and CD19 in flow cytometry. The control group showed fibroblast-like morphology on day 1 (Fig. 1). The shapes of the cells in 0.001 and 0.01 μg/mL of A. radix were similar to that of the untreated control group. The morphology of the cells on day 3 is shown in Figure 2. The shapes of the cells in 0.001 and 0.01 μg/mL were similar to that of the untreated control group.
Evaluation of cell morphology on day 1.
The control group showed fibroblast morphology on day 1. The shapes of the cells in 0.001 and 0.01 μg/mL of Asiasari radix were similar to that of the untreated control group.
A : Control group
B : 0.001 μg/mL of Asiasari radix
C : 0.01 μg/mL of Asiasari radix
2. Cell proliferation
The results of cell proliferation on days 1 and 3 are shown in Figures 3 and 4, respectively. The cultures that were growing in the presence of A. radix on day 1 showed an increase in the CCK-8 value. The relative values of CCK-8 assays of 0.001 and 0.01 μg/mL of A. radix are 130.6 % ± 1.8 % and 129.3 % ± 1.5 %, respectively, when the CCK-8 result of the untreated control group on day 1 is considered 100% (100.0 % ± 21.9 %) (P = 0.051). The results on day 3 are shown in Figure 4.
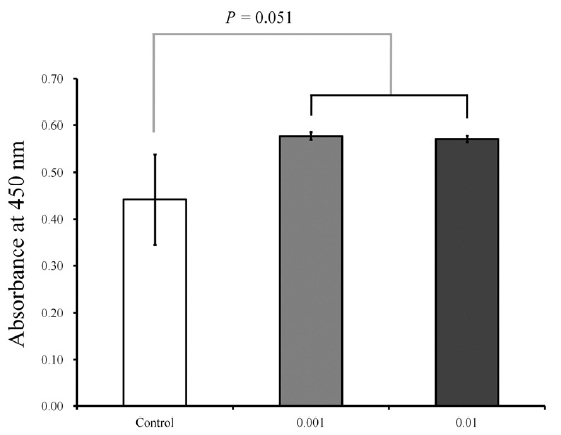
Cellular viability on day 1.
The cultures that were growing in the presence of Asiasari radix on day 1 showed an increase in the CCK-8 value. The relative values of CCK-8 assays of 0.001 and 0.01 μg/mL of Asiasari radix are 130.6 % ± 1.8 % and 129.3 % ± 1.5 %, respectively, when the CCK-8 result of the untreated control group on day 1 is considered 100% (100.0 % ± 21.9 %) (P = 0.051).
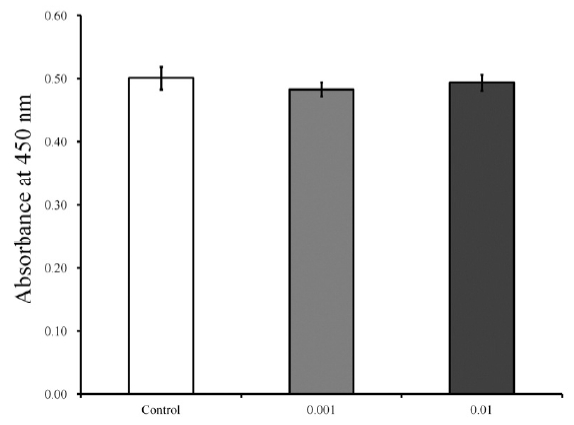
Cellular viability on day 3.
The cultures that were growing in the presence of 0.001 and 0.01 μg/mL of Asiasari radix did not show any statistically significant changes in the CCK-8 assays on day 3 (P = 0.288). The relative values of 0.001 and 0.01 μg/mL groups are 96.5 % ± 2.0 % and 98.6 % ± 2.5 %, respectively, when the CCK-8 results of the untreated control group on day 3 is considered 100 % (100.0 % ± 3.5 %)
The cultures that were growing in the presence of 0.001 and 0.01 μg/mL of A. radix did not show any statistically significant changes in the CCK-8 values on day 3 (P = 0.288). The relative values of 0.001 and 0.01 μg/mL groups are 96.5 % ± 2.0 % and 98.6 % ± 2.5 %, respectively, when the CCK-8 results of the control group on day 3 is considered 100 % (100.0 % ± 3.5 %).
Discussion
In this report, we examined the effects of a low concentration of the extract of Asiasari radix on the morphology and proliferation of the human mesenchymal stem cells that were derived from periodontal tissue. Low concentrations (0.001 and 0.01 μg/mL) of A. radix seemed to increase the proliferation of the stem cells derived from the gingiva.
Previous studies evaluating the effects of A. radix on cell proliferations showed conflicting results. Some showed no significant changes at the range of concentrations (0.0001 to 1,000 μg/mL)4,7), but others showed repressed cell proliferation in a dose-dependent manner8). However, in another report, the A. radix extract at a dose of 0.1 μg/mL increased the proliferation5). The different responses to A. radix may be explained in part, by the type of cells, culture condition, or culture period3,12).
Mesenchymal stem cells derived from gingival connective tissue have the capacity to be differentiated into osteoblasts, chondroblasts, and adipocytes13), and have immunomodulatory anti-inflammatory and regenerative properties14–16). They are relatively homogenous and they proliferate fast and they are reported to have easy, relatively noninvasive access17–18). Moreover, gingiva-derived mesenchymal stem cells were suggested to be superior to bone marrow-derived mesenchymal stem cells in that they do not lose the MSC characteristic at higher passages, maintain a normal karyotype and exhibit telomerase activity in long-term cultures18). These stem cells may be very useful in the research field and in the treatment of disease3,13). Stem cells derived from the gingiva have been applied for the treatment of rheumatoid arthritis, colitis, and allergic contact dermatitis15,19,20), bony and cutaneous wound healing21,22). There also is a study demonstrating new bone growth from the transplanted gingiva-derived stem cells in the calvarial and the mandibular rat defect22). Gingiva-derived stem cell spheriods were fabricated using concave microwells and the shape and the viability were maintained during the experimental periods23). It can be suggested that gingiva-derived stem cells represent an accessible candidate for regenerative therapies24)
Various methods are available for the assessment of cellular proliferation25–30). Bradford assay may be used as an indirect measurement of cell proliferation because it measures the content of viable cells by measuring total protein concentration26). Trypan blue assay is a dye exclusion test that is used to determine the number of viable cells because live cells possess intact cell membranes that exclude the dyes25). The MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay may be more sensitive than tryphan blue because it assesses the cell proliferation through the determination of mitochondrial dehydrogenase activity27). However, an additional step is needed for the MTT assay; this step requires the water insoluble formazan salt to be solubilized for the assay28). The tetrazolium dyes MTS and XTT form a soluble formazan that can be measured directly without an additional solubilization step29). CCK-8 assay used in this study relies on the ability of mitochondrial dehydrogenases to oxidize WST-8 [2-(2-methoxy -4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H tetrazolium, monosodium salt] into a formazan product30). In this study, the CCK-8 assay was used because the detection sensitivity of CCK-8 is higher than other assays using MTT, XTT or MTX tetrarazolium dyes and tetrazolium dye WST-8 in CCK-8 assay is reported to be less toxic to the tested cells31–32). Proliferation may also be assessed with the assay, which is based on the detection of 5′-bromo-2′-deoxy-uridine (BrdU) that is incorporated into the DNA of proliferating cells in place of thymidine32).
Within the limits of this study, low concentrations of A. radix seemed to increase the proliferation of the stem cells that were derived from the gingiva and did not have adverse effects on the morphology of the cells.
Acknowledgement
This paper was supported by the Semyung University Research Grant of 2015. The authors report no conflicts of interest related to this study.