The Effects of Haedoksamul-tang on Oxidative Stress and Hyperlipidemia in LPS-induced ICR Mouse
Article information
Abstract
Objectives:
The present study was conducted to examine whether Haedoksamul-tang (HS), a traditional oriental herbal medicine, have beneficail effects on anti-inflammation and dyslipidemia in lipopolysaccharide (LPS)-induced ICR mouse.
Methods:
Twenty four 8-week old male ICR mouse were divided into four groups: normal untreated; LPS treatment only; HS 10 mg/kg plus LPS treatment; and HS 30 mg/kg plus LPS treatment. HS was orally administered per day for 2days. Twenty-four hours after LPS injection (10 mg/kg/day, i.p.), all the mice were sacrificed, and serological changes were evaluated. The levels of nuclear factor-κB (NF-κB), sterol regulatory element-binding transcription protein 1 (SREBP-1) activity and cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), tumor necrosis factor a (TNF-a), monocyte chemotactic protein 1 (MCP-1), acetyl-CoA carboxylase a (ACCa) expression were analyzed in Western blot analysis.
Results:
HS inhibited oxidative stress in the liver of LPS-induced ICR mice. The LPS-induced ICR mice exhibited the increase of NF-κB activity and COX-2, iNOS, TNF-a, MCP-1 expressions in the liver, while HS treatment significantly inhibited them. Moreover, The administration of HS significantly decreased the elevated serum triglyceride and down-regulated the levels of SREBP-1, ACCa in the liver of LPS-induced ICR mice.
Conclusions:
In conclusion, HS could have hepato-protective effects against the oxidative stress-related inflammation and abnormal lipid metabolism.
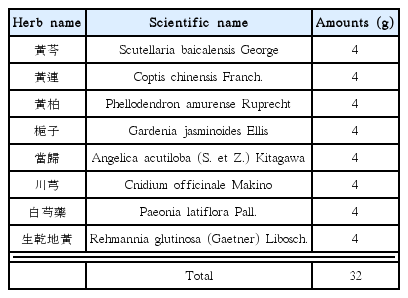
Inhibition effects of HS on serum and hepatic oxidative stress in LPS-induced ICR mice. Serum ROS (A), hepatic ROS (B), hepatic TBARS (C) levels. N: normal group, Veh: vehicle-treated mice, HS10: HS 10 mg/kg treated mice, HS30: HS 30 mg/kg treated mice. Bars represent means ± SD. ** P < 0.01 versus vehicle-treated mice values.
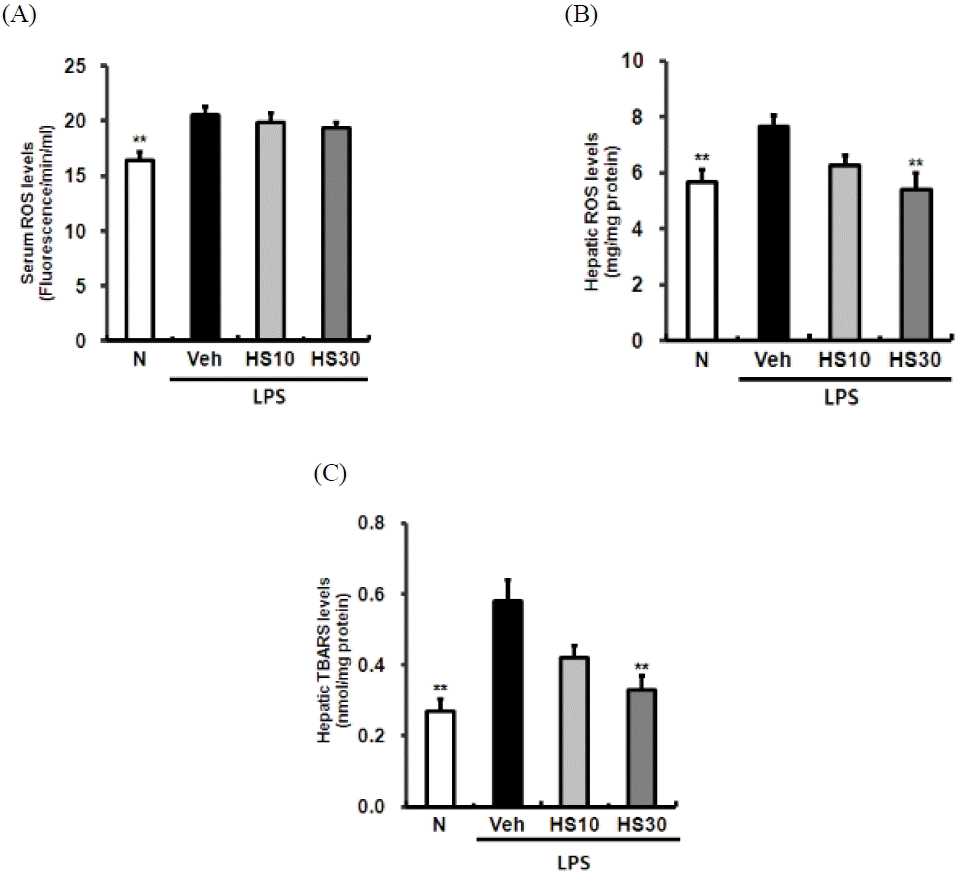
Effects of HS on NF-κBp65 activity in LPS-induced ICR mice liver. N: normal group, Veh: vehicle-treated mice, HS10: HS 10 mg/kg treated mice, HS30: HS 30 mg/kg treated mice. Histone was used for loading control. Bars represent means ± SD. * P < 0.05, *** P < 0.001 versus vehicle-treated mice values.
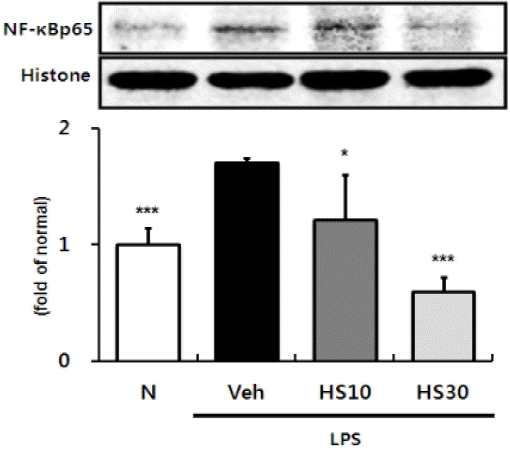
Effects of HS on COX-2 and iNOS expressions in LPS-induced ICR mice liver. COX-2 (A) and iNOS (B) protein expressions in liver. N: normal group, Veh: vehicle -treated mice, HS10: HS 10 mg/kg treated mice, HS30: HS 30 mg/kg treated mice. ß-actin was used for loading control. Bars represent means ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001 versus vehicle-treated mice values.
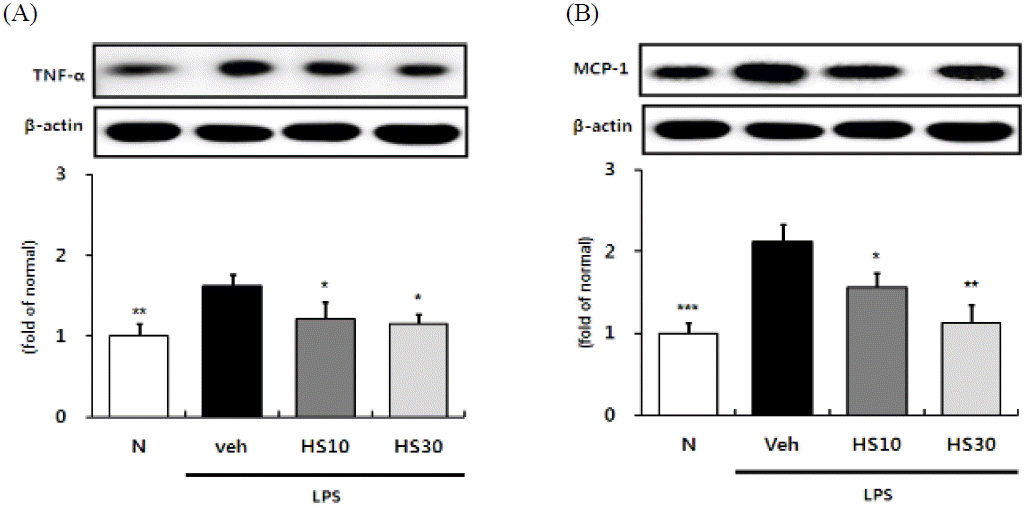
Effects of HS on TNF-a and MCP-1 expressions in LPS-induced ICR mice liver. TNF-a (A) and MCP-1 (B) protein expressions in liver. N: normal group, Veh: vehicle-treated mice, HS10: HS 10 mg/kg treated mice, HS30: HS 30 mg/kg treated mice. ß-actin was used for loading control. Bars represent means ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001 versus vehicle-treated mice values.
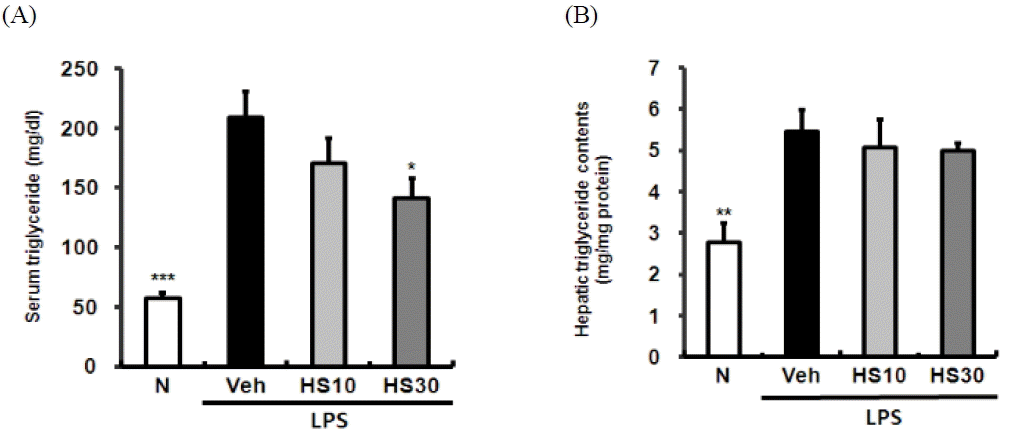
Effects of HS on serum and hepatic triglyceride levels in LPS-induced ICR mice. Serum triglyceride (A), hepatic triglyceride (B) levels. N: normal group, Veh: vehicle-treated mice, HS10: HS 10 mg/kg treated mice, HS30: HS 30 mg/kg treated mice. Bars represent means ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001 versus vehicle-treated mice values.
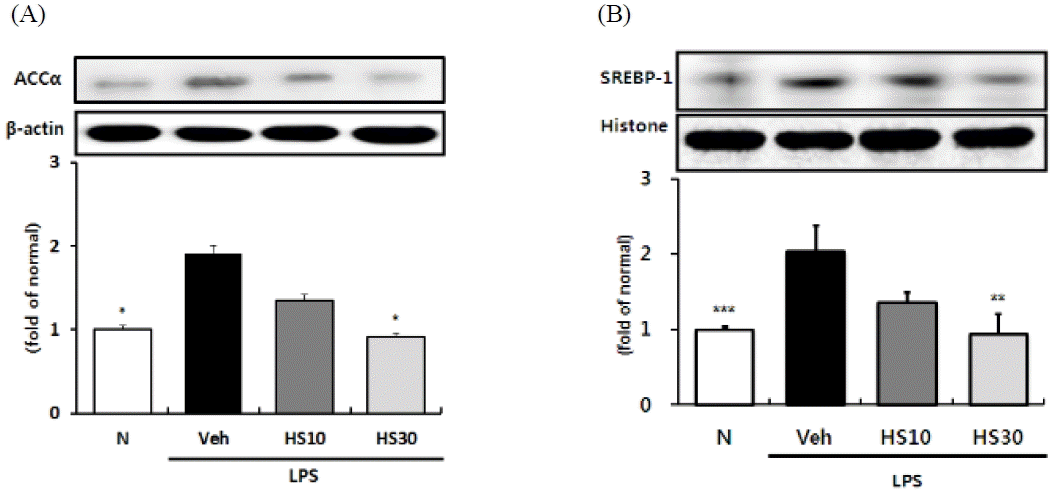
Effects of HS on SREBP-1 activity and ACCa expression in LPS-induced ICR mice liver. SREBP-1 (A) activity and ACCa (B) protein expression in liver. N: normal group, Veh: vehicle-treated mice, HS10: HS 10 mg/kg treated mice, HS30: HS 30 mg/kg treated mice. Histone or ß-actin was used for loading control. Bars represent means ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001 versus vehicle-treated mice values.