Anti-atopic dermatitis effects of Poncirus trifoliata Rafinesque via regulation of immune response and nerve growth factor
Article information
Abstract
Objectives:
Poncirus trifoliata Rafinesque has been known to have anti-allergic effects in skin diseases. However, anti-atopic dermatitis effects of P. trifoliata Rafinesque have not been studied yet in skin diseases. The present study evaluated the anti-atopic dermatitis effects of P. trifoliata Rafinesque (PTR) using external treatments on AD.
Methods:
AD lesions were induced by the repeated application of 2, 4-Dinitrochlorobenzene (DNCB) on the shaved back of BALB/c mice. 100 μℓ of PTR extracts was applied to the AD lesions for 11 days. Histological assessments, mast cells count and serum levels of IgE were analyzed. The anti-pruritic effects of PTR were examined by the change of scratching frequency and nerve growth factor (NGF) expression. In addition, the anti-inflammatory effects of PTR were examined by the expressions of Th2/Th1 cytokines and pro-inflammatory in dorsal skin.
Results:
Histopathological findings showed that topical application of PTR decreased the thickness of dermal and epidermal skin compared with the DNCB group. PTR also notably decreased the mast cells count and serum IgE. The scratching behavior of mice and expression of NGF were significantly reduced. In addition, PTR group significantly suppressed the IL-4, IL-6, IFN-γ and TNF-α cytokines compared to the DNCB group.
Conclusions:
These results indicated that P. trifoliata Rafinesque possess anti-pruritus and anti-atopic dermatitis properties. Therefore, P. trifoliata Rafinesque might be used for treatment of pruritus and atopic dermatitis.
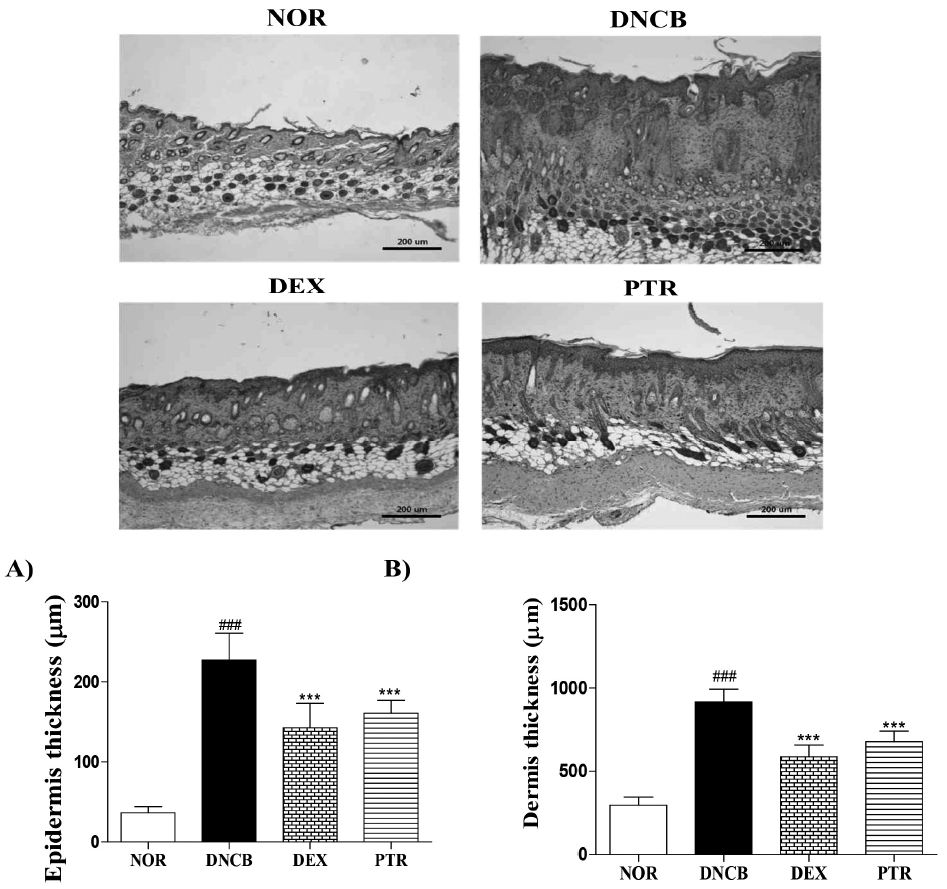
Effects of PTR on skin thickness in DNCB-induced mice. (A) The thickness of epidermis, (B) the thickness of dermis. Hematoxylin-eosin staining showed a decrease thickness of epidermis and dermis in the PTR-treated group. Results shown are representative of five observations. Data are presented as means ± SD and analyzed with ANOVA. ### P < 0.001, versus the NOR group. *** P < 0.001, versus the DNCB group. Magnification: X 100.
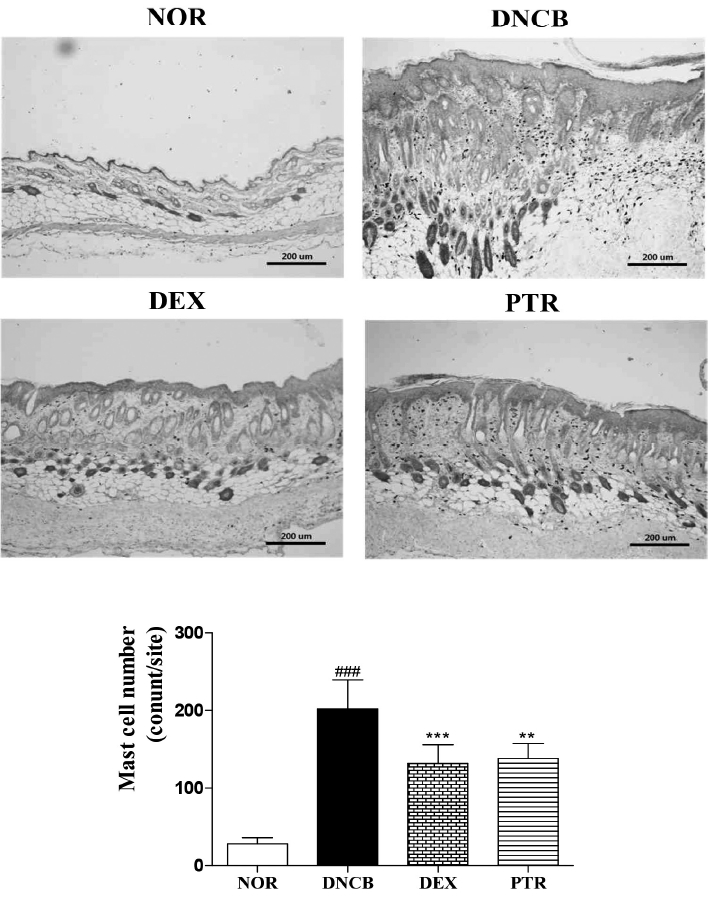
Effects of PTR on the number of mast cells in DNCB-induced mice. Toluidine blue staining showed a decrease the number of mast cells in the RTR group. Results shown are representative of five observations. Data are presented as means ± SD and analyzed with ANOVA. ### P < 0.001, versus the NOR group. *** P < 0.001, ** P < 0.01 versus the DNCB group. Magnification: X 100.
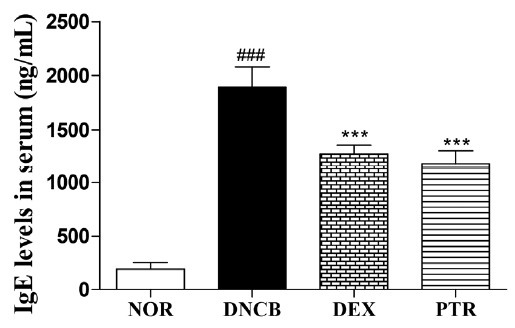
Effects of PTR on serum IgE level in DNCB-induced mice. Data are presented as means ± SD and analyzed with ANOVA ### P < 0.001, versus the NOR group. *** P < 0.001, versus the DNCB group.
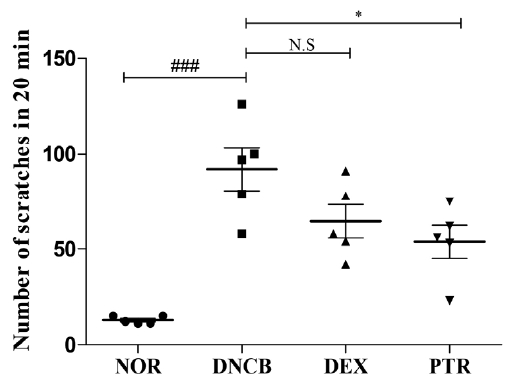
Effects of PTR on scratching behavior in DNCB-induced mice. Scratching behavior of mouse was checked after 1 h had passed following the last application of DNCB (on 18 day). Each mouse of all group were videotaped for 20 min to count the number of scratching behavior. Data are presented as means ± SD (n = 5) and analyzed with ANOVA. ### P < 0.001, versus the NOR group. * P < 0.05, versus the DNCB group. N.S: non-significant.
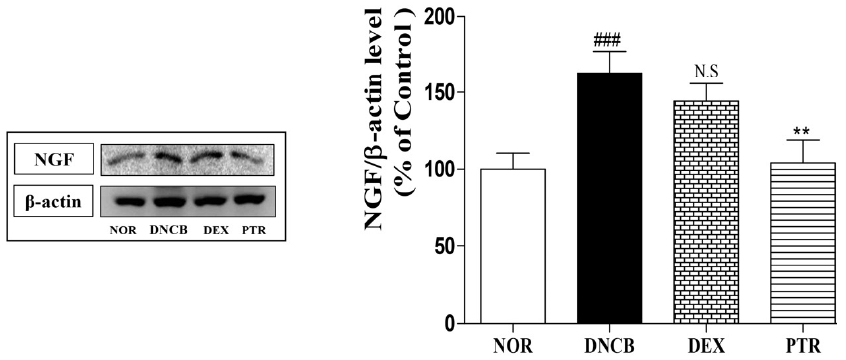
Effects of PTR on NGF expression in DNCB-induced mice. The expression of NGF was confirmed by western blotting assay. ### P < 0.001, versus the NOR group. ## P < 0.01, versus the DNCB group. N.S: non-significant.
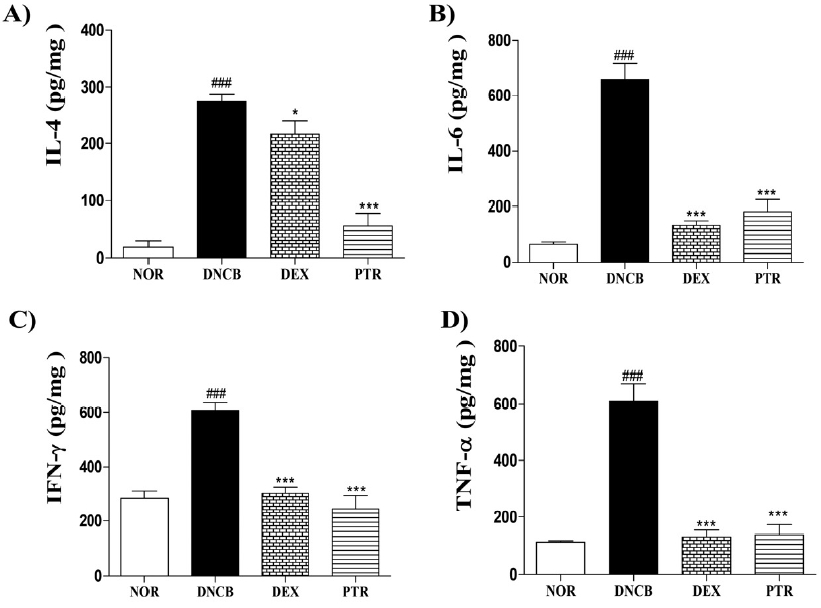
Effects of PTR on levels of cytokine in DNCB-induced mice. The cytokine levels of IL-4, −6, IFN-γ and TNF-α were measured in the dorsal skin using mouse ELISA kits. Data are presented as means ± SD (n = 5) and analyzed with ANOVA. ### P < 0.001, versus the NOR group. *** P < 0.001 * P < 0.05, versus the DNCB group.
