Survey Research for Developing Educational Programs on Clinical Trials of Korean Medicine Devices
Article information
Abstract
Objectives:
The purpose of this study was to investigate and analyze the demands for educational programs on clinical trials of Korean medicine devices, and develop training programs based on the needs of Korean medicine.
Methods:
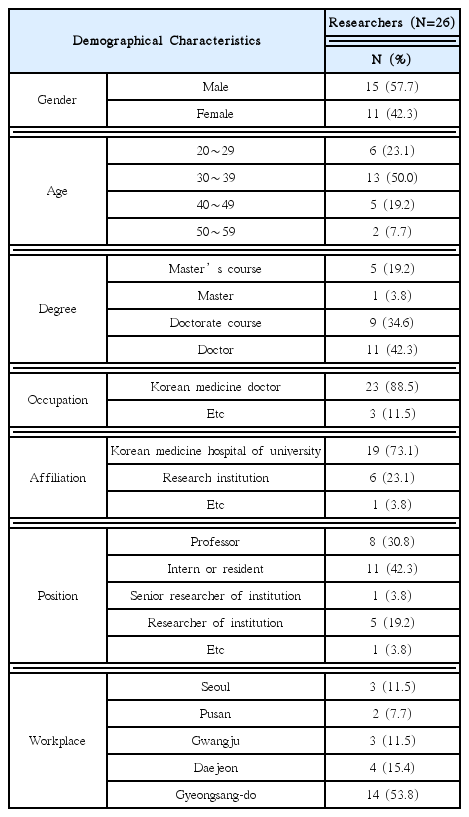
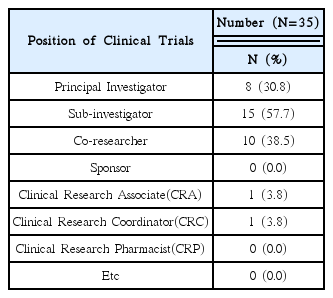
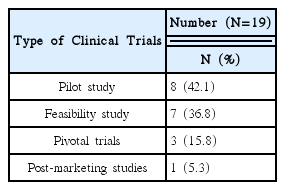
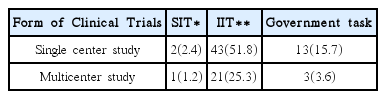
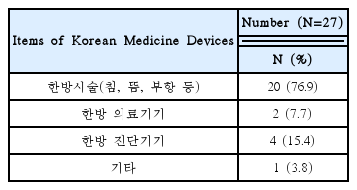
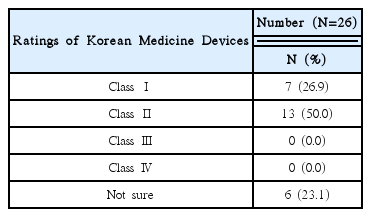
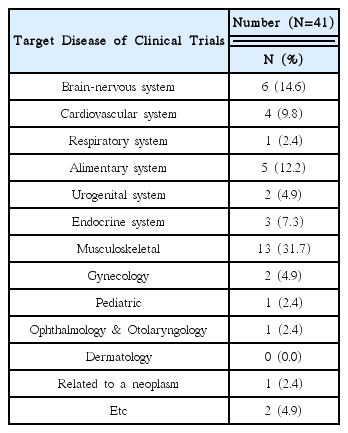
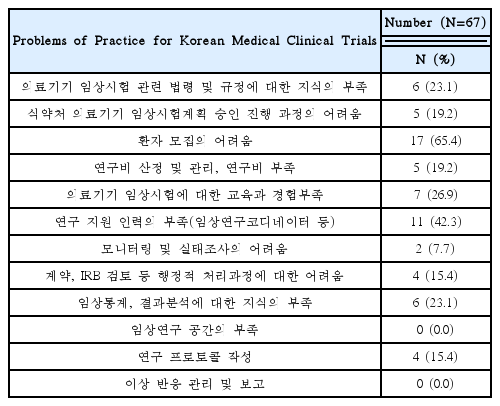
This research was conducted targeting 26 volunteer applicants who had participated in clinical trials of Korean medicine devices within the last five years (2010–2015). The survey was carried out between May 1, 2015 and May 26, 2015 via e-mail. After receiving questionnaire replies, the material was established. Using obtained data, frequency analyses were performed using SPSS 20.0 version.
Results:
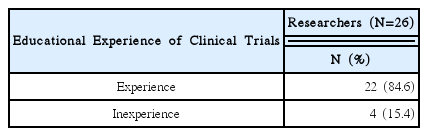
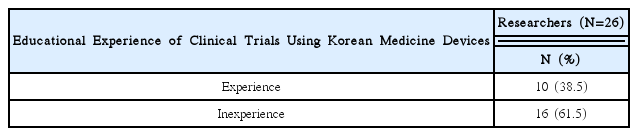
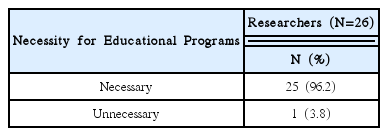
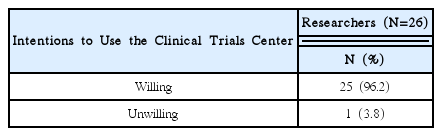
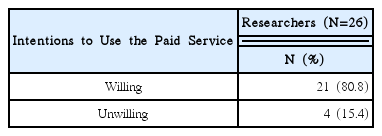
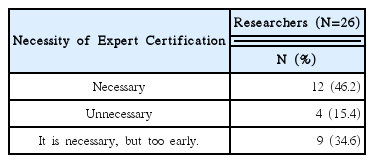
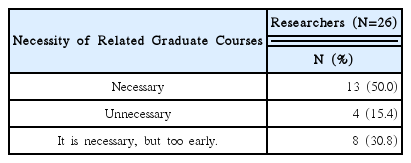
92% of the researchers who participated in the survey expected introduction of educational programs on clinical trials and anticipated that programs contain information that can meet the needs of each researcher. In addition, according to the analysis, introducing expert certification for clinical trials of Korean medicine devices is necessary, and offering related graduate courses are also needed.
Conclusions:
As a result of this study, researchers had difficulties during clinical trials of Korean medicine devices. If the educational programs were to be developed and institutional frameworks support them effectively, it would prove to be helpful to researchers in clinical trials.
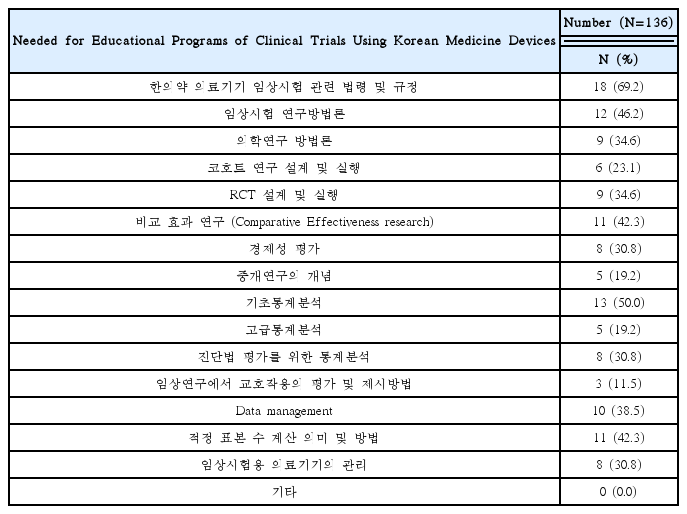
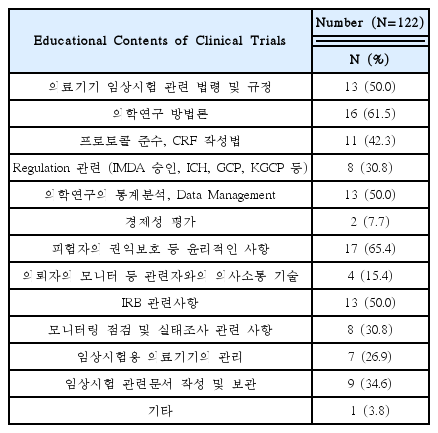
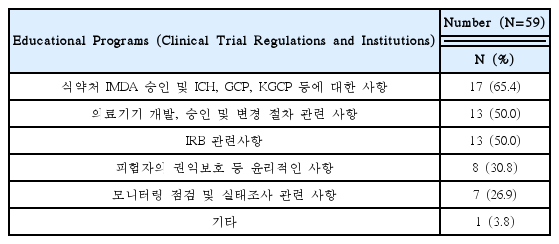
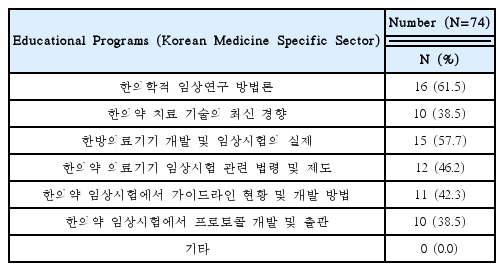
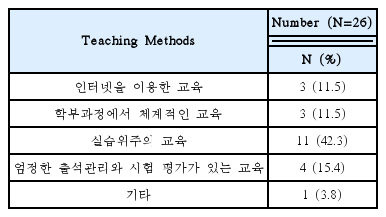
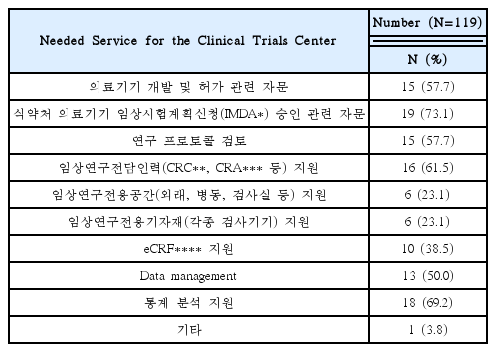
Needed for Educational Programs of Clinical Trials Using Korean Medicine Devices(Multiple Responses Possible)