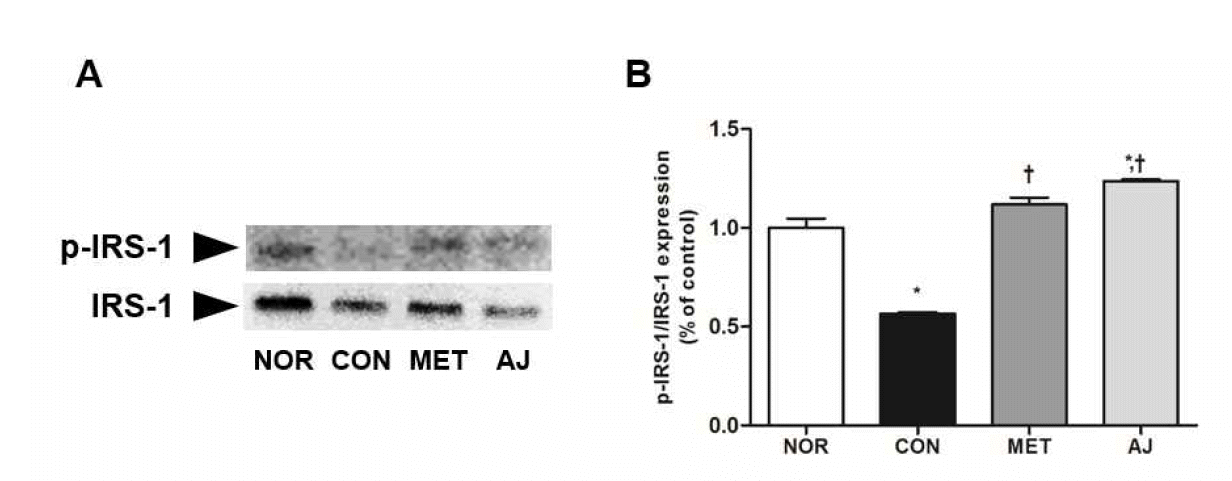
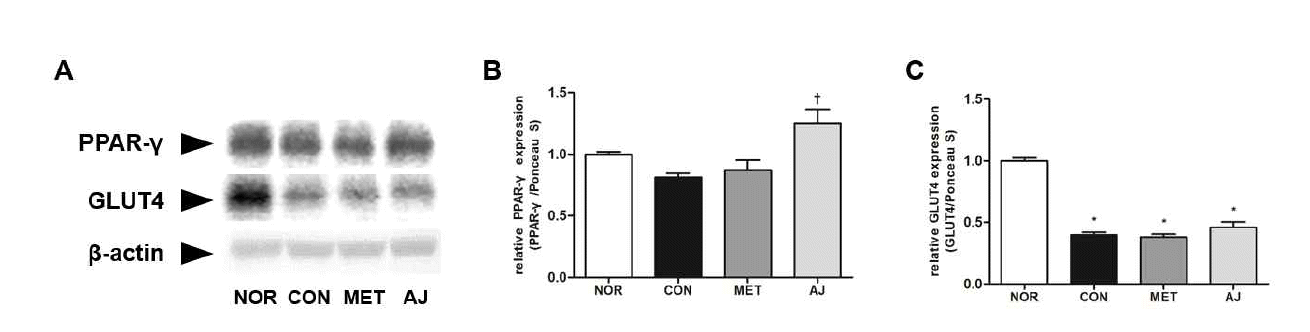
References
1. Mccarthy MI. Genomics, type 2 diabetes, and obesity. The New England Journal of Medicine 2010;363(24):2339–2350.
2. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444(7121):840–846.
3. Korean diabetes association. Korea centers for disease control and prevention. Diabetes fact sheet in korea 2012.
4. Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabetic medicine: a journal of the British Diabetic Association 1998;15(7):539–553.
5. American diabetes association. Standards of medical care in diabetes-2014. Diabetes Care 2014;37(1):S14–S80.
6. Cheng AYY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Canadian Medical Association journal 2005;172(2):213–226.
7. Yale JF. Oral antihyperglycemic agents and renal disease: New agents, new concepts. Journal of the American Society of Nephrology 2005;16:S7–S10.
8. Doo HK. Internal Medicine of Kidney System. Seongbosa 2003;:1131.
9. Kang SB, Kim GC. A pathological approach about symptoms of So-gal. The Journal of East-West Medicines 1998;23(4):21–40.
10. Kang SB. The comparative study between the transformations of sogal and the complications of diabetes mellitus. The Journal of Korean Oriental Medical Society 1998;19(2):137–152.
11. Editing commission of herbal medicine. Herbal medicine. Youngrimsa 2007;:331–332.
12. Ahn DK. Illustrated guide to korean herbal medicine. Kyohaksa 2008;:384.
13. Heo J. Donguibogam. Bubinmunhwasa 2007;:1952.
14. Choi KH, Jeong SI, Lee JH, Hwang BS, Kim SJ, Lee S, et al. Pharmacological mechanism responsible for the Atractylodes japonica-induced distal colonic contraction in rats. Phytomedicine 2011;18:408–413.
15. Han YK, Jung HW, Park YK. The roots of Atractylodes japonica Koidzumi promote adipogenic differentiation via activation of the insulin signalong pathway in 3T3-L1 cells. BMC Complementary and Alternative Medicine 2012;12:154.
16. Han HK, Yoon SJ, Kim GH. Effects of compositae plants on plasma glucose and lipid level in streptozotocin induced diabetic rats. Journal of Korean Food Science and Nutrition 2009;38(6):674–682.
17. Chen F, Xiong H, Wang J, Ding X, Shu G, Mei Z. Antidiabetic effect of total flavonoids from Sanguis draxonis in type 2 diabetic rats. Journal of ethnopharmacology 2013;149(3):729–736.
18. Vogeser M, König D, Frey I, Predel HG, Parhofer KG, Berg A. Fasting serum insulin and the homeostasis model of insulin resistance (HOMA-IR) in the monitoring of lifestyle interventions in obese persons. Clinical Biochemistry 2007;40(13–14):964–968.
19. Catalano PM, Presley L, Minium J, Mouzon SHD. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009;32(40):1076–1080.
20. Han YK, Park YK. Effect of Atractylodis Rhizoma Alba water extract on streptozotocin-induced diabetes in rats. Journal of Korean Herbology 2011;26(4):23–30.
21. Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. The Lancet 2011;378(9):169–181.
22. Adefegha SA, Oboh G, Adefegha OM, Boligon AA, Athayde ML. Antihyperglycemic, hypolipidemic, hepatoprotective and antioxidative effects of dietary clove (Szyzgium aromaticum) bud powder in a high-fat diet/streptozotocin-induced diabetes rat model. Journal of the science of food and agriculture 2014;94(13):2726–2737.
23. Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, et al. Exercise and Type 2 Diabetes. Medicine and Science in Sports and Exercise 2010;42(12):2282–2303.
24. Hong SM, Park JH, Lim YH, Park YS, Kim DS, Choi WH, et al. The relationship between diabetic retinopathy and macrovascular complication in patients with type 2 diabetes. The Korean Journal of Medicine 2011;81(3):351–358.
25. Chae HJ, Lee IS, Moon HY. Effects of Schizandra Cchinensis fruit extract on the hyperglycemia and hyperlipemia in streptozotocin-induced diabetic rats. Korean Society for Biotechnology and Bioengineering Journal 2011;26(2):126–130.
26. Choi ES, Rhee EJ, Kim JH, Won JC, Park CY, Lee WY, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and future risk of diabetes mellitus in korean men. Diabetes and Metabolism Journal 2008;32(6):498–505.
27. Han JS, Back SH, Hur JU, Lin YH, Gildersleeve R, Shan J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nature cell biology 2013;15(5):481–490.
28. Dor Y, Glaser B. Beta-cell differentiation and type 2 diabetes. The New England Journal of Medicine 2013;368(6):572–573.
29. Imamura F, Mukamal KJ, Meigs JB, Luchsinger JA, Ix JH, Siscovick DS, et al. Risk factors for type 2 diabetes mellitus preceded by beta-cell dysfunction, insulin resistance, or both in older adults. American Journal of Epidemiology 2013;177(12):1418–1429.
30. Ryu HS, Park SY, Ma D, Zhang J, Lee W. The Induction of MicroRNA Targeting IRS-1 Is Involved in the Development of Insulin Resistance under Conditions of Mitochondrial Dysfunction in Hepatocytes. PloS one 2011;6(3):e17343.
31. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001;414(6865):799–806.
32. Hemi R, Yochananov Y, Barhod E, Kasher MM, Karasik A, Tirosh A, et al. p38 mitogen-activated protein kinase-dependent transactivation of ErbB receptor family: a novel common mechanism for stress-induced IRS-1 serine phosphorylation and insulin resistance. Diabetes 2011;60(4):1134–1145.
33. Ohshima K, Mogi M, Jing F, Iwanami J, Tsukuda K, Min LJ, et al. Direct angiotensin II type 2 receptor stimulation ameliarates insulin resistance in type 2 diabetes mice with ppar γ activation. PloS one 2012;7(11):e48387.
34. Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annual review of nutrition 1996;16:235–256.
35. Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. The New England Journal of Medicine 1999;341(4):240–246.
36. Kainulainen H, Breiner M, Schurmann A, Marttinen A, Virjo A, Joost HG. In vivo glucose uptake and glucose transporter proteins GLUT1 and GLUT4 in heart and various types of skeletal muscle from streptozotocin-diabetic rats. Biochimica et Biophysica Acta 1994;1225(3):275–282.