Herbal-drug-associated Adverse Events Reported in the Internet Newspaper Articles
Article information
Abstract
Objectives:
The aim of this study was to understand the characteristics of herbal-drug-associated adverse events (AEs) reported in the internet newspaper articles and to take a countermeasure against the safety issue of herbal drugs.
Methods:
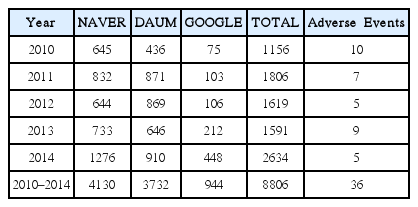
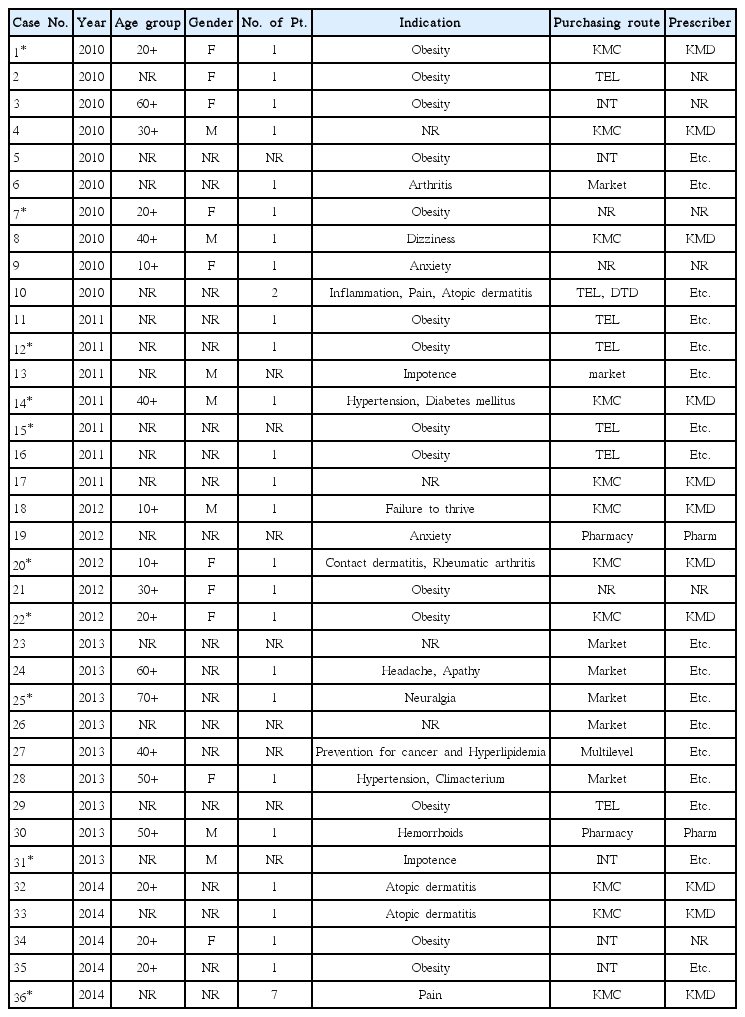
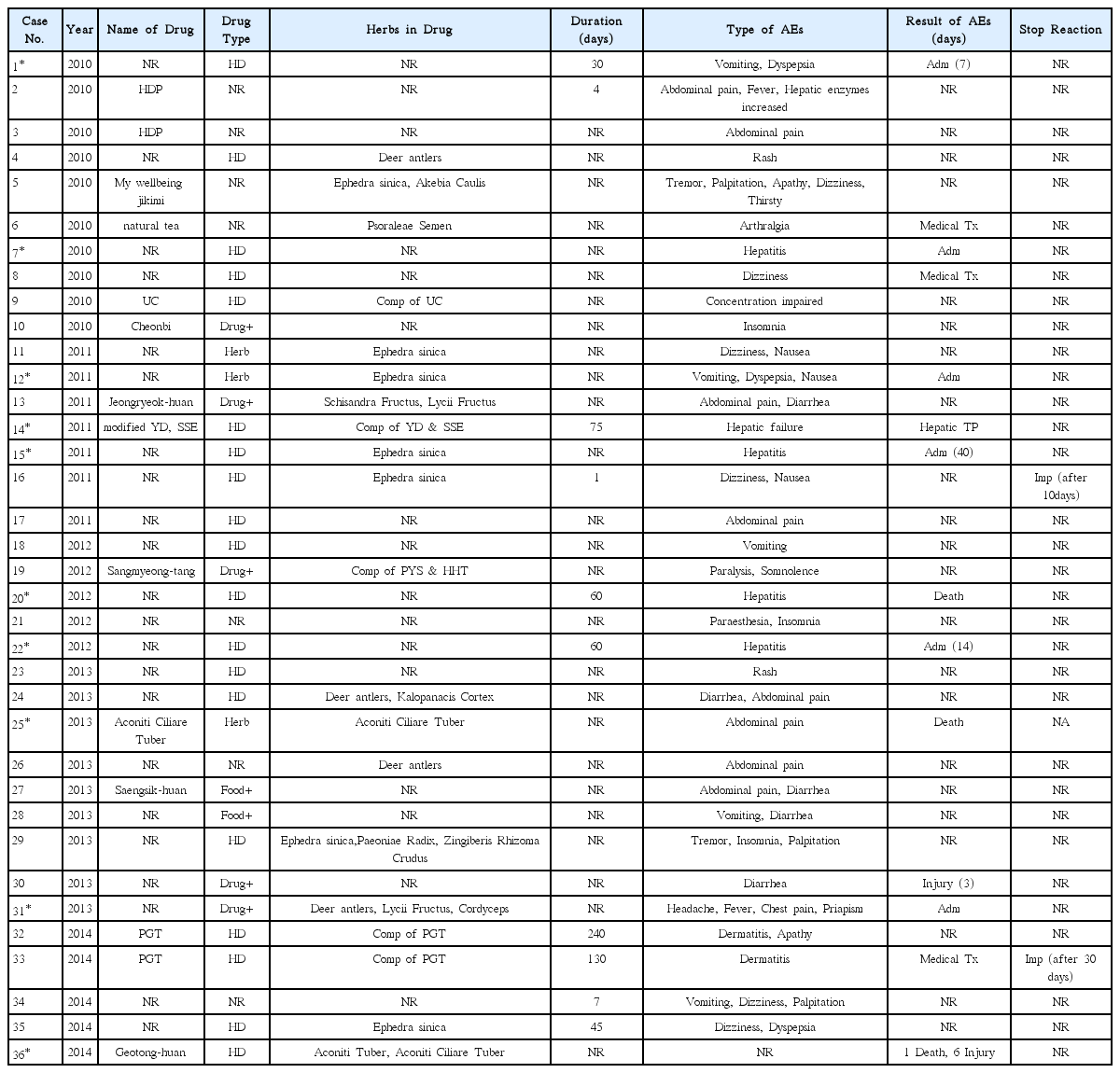
We searched the internet newspaper articles published from 2010 to 2014 in the 3 major portal sites in Korea, NAVER, DAUM, and GOOGLE. Search terms were the Korean words equivalent of ‘herbal drug’ and ‘side effects’. Informations on the type and characteristics of suspected herbal drugs, AEs, and the patient records were extracted from the articles reporting the herbal-drug-associated AE occurred in Korea.
Results:
From 8,806 articles, a total of 36 AEs were found. The most frequently reported age group was 20s, and women outnumbered men. Obesity was the most common cause of administration. Doctors of Korean medicine clinic were the most commonly referred prescribers and purchasing route (11 cases). The most frequently mentioned medicinal herb was Ephedra sinica (7 cases) and the most commonly reported AEs were abdominal pain (8 cases), dizziness (6 cases), diarrhea (5 cases), and vomiting (5 cases) were followed in order. Ten cases were judged as serious AEs, and the others were not.
Conclusions:
Current customers demand health care providers to offer them sufficient information on the safety of herbal drugs. To satisfy their requirements, physicians of Korean medicine should be able to explain, predict, prepare, recognize, and deal with the herbal-drug-associated AEs. We propose an establishment of pharmacovigilance system for herbal medicine, in which doctors of Korean medicine are participated as important personnel, to collect and analyze the related AEs and offer credible information on the safety of herbal drug.