Effects of Inhalable Microparticles of Socheongryong-tang on Chronic Obstructive Pulmonary Disease in a Mouse Model
Article information
Abstract
Objectives:
This study aimed to evaluate the effects of microparticles of Socheongryong-tang (SCRT) on chronic obstructive pulmonary disease (COPD) in a mouse model.
Methods:
The inhalable microparticles containing SCRT were produced by spray-drying with leucine as an excipient, and evaluated with respect to the aerodynamic properties of the powder by Andersen cascade impactor (ACI). Its equivalence to SCRT extract was evaluated using lipopolysaccharide (LPS) and a cigarette-smoking (CS)-induced murine COPD model.
Results:
SCRT microparticles provided desirable aerodynamic properties (fine particle fraction of 49.6±5.5% and mass median aerodynamic diameter of 4.8±0.3 μm). SCRT microparticles did not show mortality or clinical signs over 14 days. Also there were no significant differences in body weight, organ weights or serum chemical parameters between SCRT microparticle-treated and non-treated groups. After 14 days the platelet count significantly increased compared with the non-treated group, but the values were within the normal range. Inhalation of SCRT microparticles decreased the rate of neutrophils in blood, granulocytes in peripheral blood mononuclear cells (PBMC) and bronchoalveolar lavage fluid (BALF) and level of TNF-α and IL-6 in BALF on COPD mouse model induced by LPS plus CS. This effect was verified by histological findings including immunofluorescence staining of elastin, collagen, and caspase 3 protein in lung tissue.
Conclusions:
These data demonstrate that SCRT microparticles are equivalent to SCRT extract in pharmaceutical properties for COPD. This study suggests that SCRT microparticles would be a potential agent of inhalation therapy for the treatment of COPD.
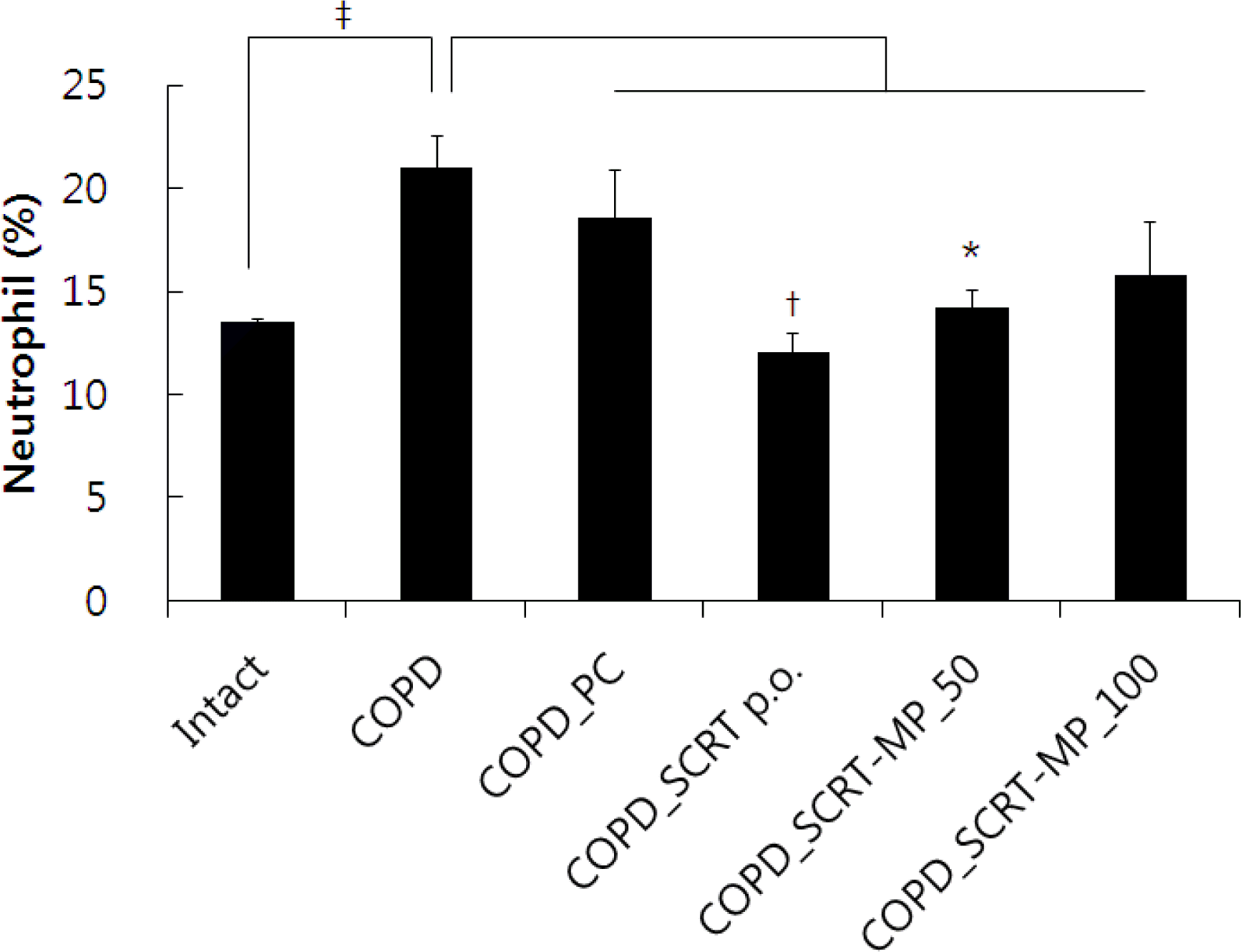
Effect of SCRT-MP on neutrophil % of blood in COPD mice. Mice were challenged by aspiration of LPS+CS, and then treated with dexamethasone (PC: positive control, 0.5 mg/kg), SCRT (160 mg/kg, p.o), SCRT microparticle (50, 100 mg/kg) for 21 days (n=4).
*: p<0.05 †: p<0.01 compared to COPD-CT by T-test.
‡: p<0.05 compared to Intact by T-test.
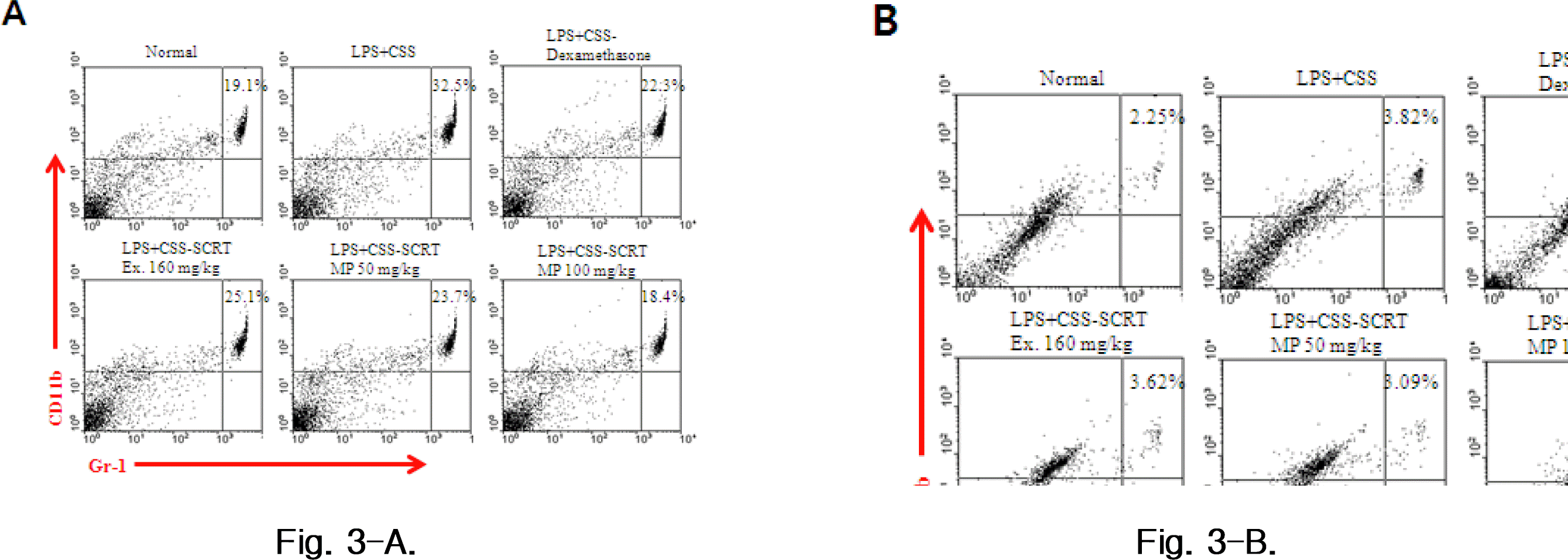
Effect of SCRT-MP on CD11b/Gr-1 cell rate of PBMC (A) and BALF (B) in COPD mice. Mice were challenged by aspiration of LPS+CS, and then treated with dexamethasone (PC: positive control, 0.5 mg/kg), SCRT (160 mg/kg, p.o), SCRT microparticle (50, 100 mg/kg) for 21 days.
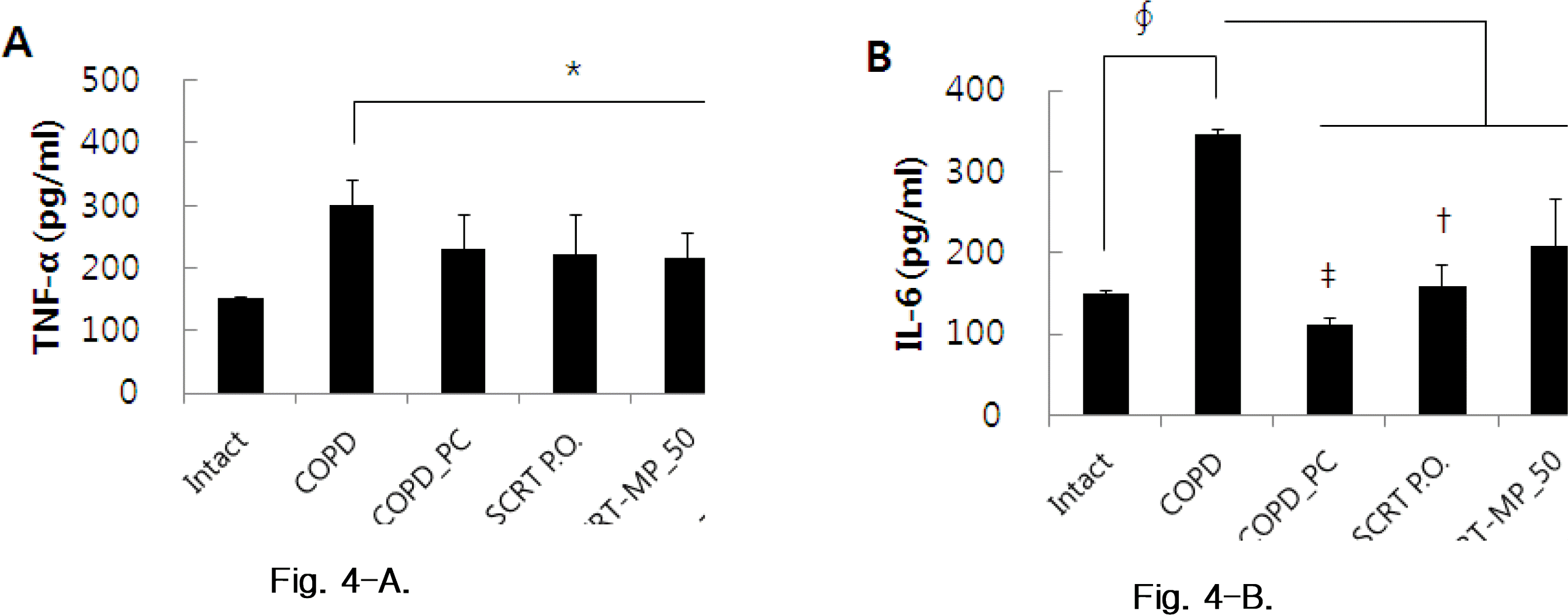
Effects of SCRT-MP on TNF-α and IL-6 production in COPD mice. Mice were challenged by aspiration of LPS+CS, and then treated with dexamethasone (PC: positive control, 0.5 mg/kg), SCRT (160 mg/kg, p.o), SCRT microparticle(50, 100 mg/kg) for 21 days (n=4). (A) TNF-α, (B) IL-6.
*: p<0.05 †: p<0.01 ‡: p<0.001 compared to COPD_CT by T-test.
∮ : p<0.001 compared to Intact by T-test.
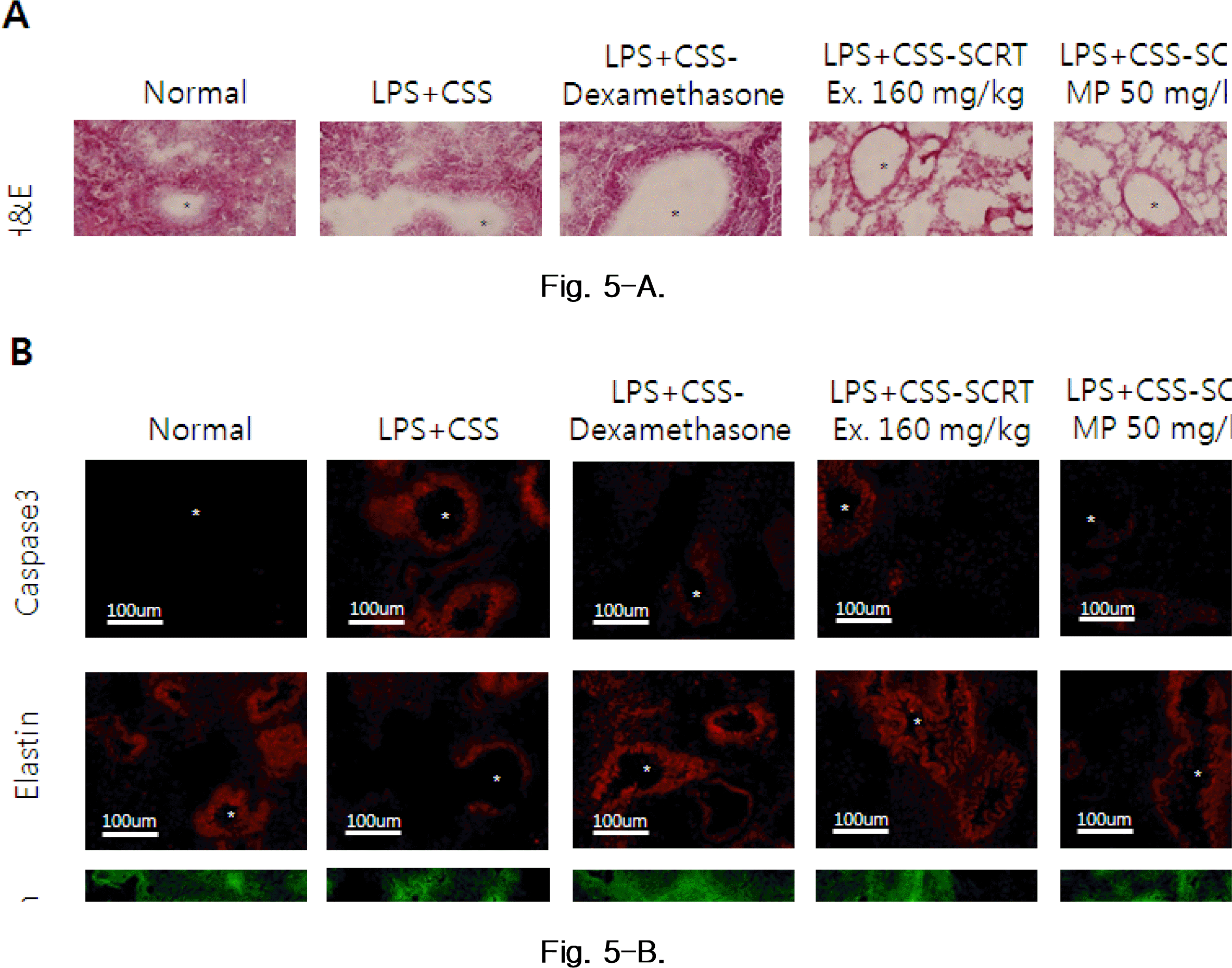
Histological analysis(A) of lung tissues. Mice were challenged by aspiration of LPS+CS, and then treated with dexamethasone (PC: positive control, 0.5 mg/kg), SCRT (160 mg/kg, p.o), SCRT microparticle (50, 100 mg/kg) for 21 days. Lung tissues were examined under microscope (200 × magnification) after H&E staining. Asterisks denote alveoli. Immunofluorescence analysis(B) for caspase 3, elastin, collagen in lung tissue. Mice were challenged by aspiration of LPS+CS, and then treated with dexamethasone (PC: positive control, 0.5 mg/kg), SCRT (160 mg/kg, p.o), SCRT microparticle(50, 100 mg/kg) for 21 days. Lung tissues were examined under microscope (200 × magnification) after immunofluorescence staining against caspase 3, elastin, collagen. Asterisks denote alveoli.
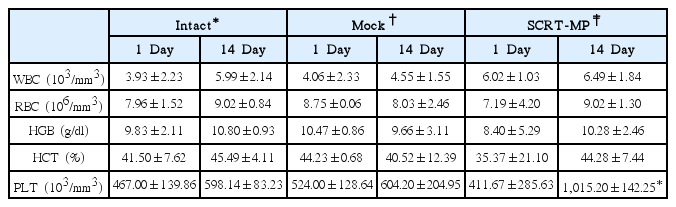
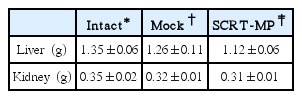
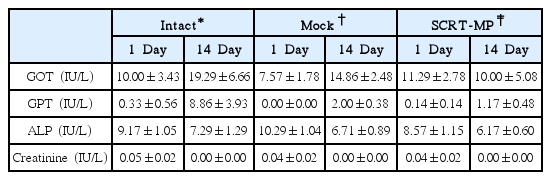
Hematological Values of Male Mice Inhaled SCRT Microparticle on 1 and 14 Day in Single Dose Toxicity Study