Lonicerae Flos Inhibits Cigarette-induced Lung Inflammatory Responses in Animal Model of Chronic Obstructive Pulmonary Disease
Article information
Abstract
Objectives:
In the present study, we evaluate the anti-inflammatory effect of Lonicerae Flos on cigarette-induced lung inflammatory responses in animal model of chronic obstructive pulmonary diseases (COPD).
Methods:
To inspect the effects of Lonicerae Flos, we evaluated Lonicerae Flos functions in vivo including immune cell profiles in bronchoalveolar lavage (BAL) fluid, cytokine production and tissue morphological changes.
Results:
Lonicerae Flos significantly inhibited immune cell infiltrations into the BAL fluid (neutrophils, macrophages, lymphocytes). TNF-α, and interleukin-6 (IL-6) were substantially decreased in the BAL fluid of Lonicerae Flos-treated mice compared with cigarette-exposed control mice. In addition, the hypertrophy of goblet cells in the epithelial cells was reduced in both Lonicerae Flos- and roflumilast-treated mice.
Conclusions:
The results of this study provide evidence that treatment with Lonicerae Flos exerts strong therapeutic effects against cigarette-induced lung inflammation in vivo. Therefore, this herbal medicine may represent a novel therapeutic agent for lung inflammation in general, as well as a specific agent for the treatment of COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is the sixth leading cause of death in the world, and the prevalence of physiologically-defined COPD in adults aged ≥ 40 years is ∼ 9 to 10%1). COPD is characterized by airway obstruction and is associated with chronic bronchitis and emphysema2). In addition, COPD is associated with progressive, not fully reversible, flow limitation and associated with an abnormal inflammatory response of the lungs to noxious particles and gases. However, cigarette smoke has been identified as the most important etiologic factor in the pathogenesis of COPD2,3).
Cigarette smoke induces the production of large number of reactive oxygen species (ROS), which disturb the balance between oxidants and antioxidants, promoting lung cell apoptosis and amplifying the inflammatory responses in the lungs4). A correlation between exposure to tobacco smoke and the increased number of neutrophils in bronchoalveolar lavage fluid (BALF) has been described in humans5) and animal models6). In mouse models of cigarette smoke-induced inflammation, it has been shown that the initial neutrophil influx is mediated largely by keratinocyte-derived chemokine (KC) and macrophage inflammatory protein (MIP)-2. In addition, elevated levels of tumor necrosis factor (TNF)-α and interleukin (IL)-1β were found in the BALF of experimental animals exposed to cigarette smoke7). Apart from neutrophils, macrophage and lymphocytes have been detected in BAL fluid, as well as in sputum of COPD patients, when compared to smokers without COPD5).
Phosphodiesterases (PDEs) are cAMP- or cGMP-specific enzymes which have a ubiquitous distribution in the human organism. Several PDE4 inhibitors were developed, including initially rolipram, cilomilast, and roflumilast. Among these, roflumilast was the first to be developed and approved for market (in Europe) as a COPD therapy8). Roflumilast (3-cyclo-propyl methoxy-4-difluoromethoxy-N-[3,5-di-chloropyrid-4-y l]-benzamide) was identified as a potent and selective PDE4 inhibitor and was demonstrated to increase levels of intracellular cAMP to a lesser extent in inflammatory cells such as neutrophils, monocytes, dendritic cells, or CD4+ T cells, and in the epithelial cells of the airways and pulmonary vessels: the most striking effects were reported in vitro with both roflumilast and its metabolite roflumilast N-oxide on leukotriene b4, reactive oxygen species, TNF-α, IFN-ɣ, or granzyme B9,10). The anti-inflammatory effects of roflumilast might also result in a reduction of airway remodeling, as demonstrated in a mouse asthma model with chronic allergen exposure in which roflumilast, given after the allergic inflammation developed, demonstrated antifibrotic effects comparable with those of dexamethasone11). Neutrophil, macrophage and CD8+ T-lymphocytes play a significant role in COPD and these cells have been shown to substantially express PDE4. Thus, substances that prevent the degradation of cAMP by inhibiting the activity of PDE4 will enhance the anti-inflammatory action of this second messenger12) However, although most people do not experience problems while taking roflumilast, side effects can still occur. Common problems include diarrhea, nausea, and weight loss. Although many drugs are use d to treat COPD, adverse effects and side effects associated with several classes of drugs such as steroids have increased the need for alternative treatments such as herbal medicines13).
The flower of Lonicera Japonica Thunb. is a traditional oriental medicine that has been used clinically for the treatment of inflammation. We previously reported that extract from Lonicerae Flos effectively attenuated LPS-induced pulmonary inflammation through inhibition of the expression of neutrophil elastase (NE), and reduction of the pro-inflammatory cytokine secretion in the lungs. Based on these suggestions, we evaluated the effect of extract from Lonicerae Flos against cigarette smoke induced lung inflammation14). In addition, experimental study has demonstrated that extract from Lonicerae Flos exhibiting strongly anti-inflammatory activity, can effectively inhibit the cigarette induced tumor necrosis factor (TNF)-α, Interleukin (IL)-6 and inducible nitric oxide (NO) production in vitro15). Luteolin, a major flavonoid of Lonicera Japonica Thunb. also protects against cigarette induced lethal toxicity by inhibiting pro-inflammatory molecule expression in murine macrophage16).
Therefore, we then confirmed that Lonicerae Flos exerted anti-inflammatory effect by inhibiting the development of pulmonary neutrophilic inflammation in a murine model of cigarette-induced chronic obstructive pulmonary disease (COPD). However, the therapeutic effects and underlying mechanisms of extract from Lonicerae Flos on pulmonary disease have not yet been evaluated. In present study, we provide evidence that extract from Lonicerae Flos has potential therapeutic effects on COPD.
Methods
1. Experimental Reagent
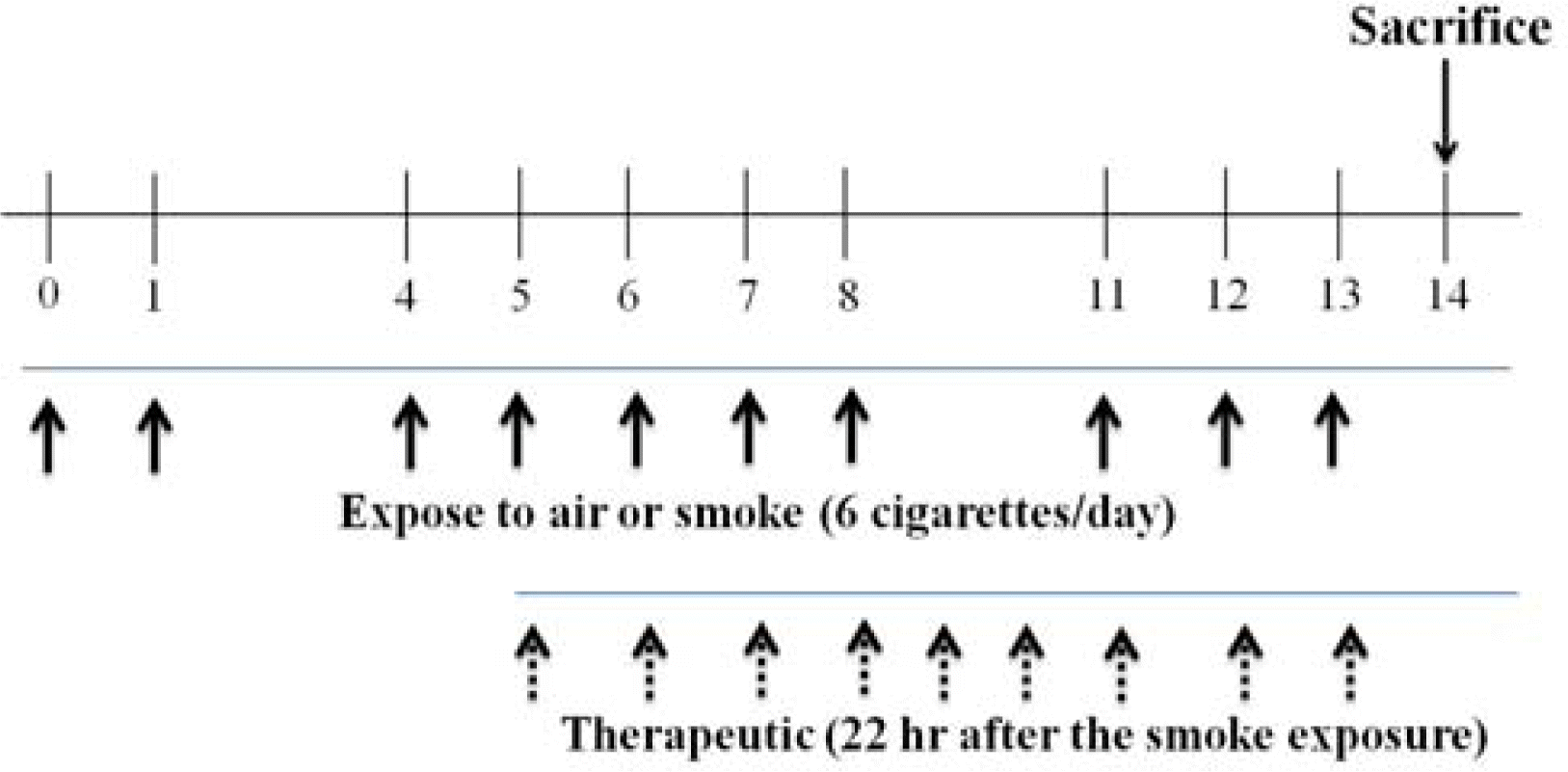
The extract of the flower of Lonicera Japonica Thunb, (Sun Ten Pharmaceutical, Taiwan) was dissolved in D-PBS to give a final concentration of 100 mg/kg. Mice were exposed to one cigarette every 5 min, for a total exposure time of 30 min. Roflumilast (Santa Cruz Biotechnology, Inc. Santa Cruz. CA., U.S.A.), which was used as a positive control, was also dissolved in 10% DMSO (5 mg/kg).
2. Cigarette induced lung inflammation model
BALB/c female mice (7 weeks of age, weigh 23-28 g, Charles River Korea, Seoul, Korea) were exposed to 3R4F reference cigarettes (University of Kentucky, Tobacco Research Institute, Lexington, KY, USA) for 2 weeks with 6 cigarettes each day on days 0, 1, 4, 5, 6, 7, 8, 11, 12 and 13. Exposure to the smoke from the cigarettes lasted for 30 min (each cigarette was completely burned in the first 1 min) and followed by exposure to laboratory air. Mice were exposed to cigarette smoke by whole body exposure, using a smoking apparatus (Cigarette of experimental, Live Cell Instrument, Seoul, South Korea) with the chamber adapted for a group of mice (chamber size=1.54 m x 0.52 m x 0.22 m). For therapeutic treatment, mice were dosed with vehicle on days 0 to 4 and Lonicerae Flos on days 5 to 13. Each group of mice was administered Lonicerae Flos via an oral injection 22 hr after cigarette exposure. The negative control group was administered non-stimulation, while mice in the positive control group were administered roflumilast via an oral injection 22 hr after cigarette exposure. Twenty-four hours after the last exposure, mice were sacrificed and BAL fluid and lung specimens collected.
During the study, the animals were housed in a pathogen-free temperature-controlled room. The institutional animal care and use committee of Kyunghee University approved the study (KHUASP(SE)-12-015).
3. Analysis of inflammation cell profiles in lung
After the animals were sacrificed, PBS (phosphate buffered saline) was slowly infused into the lungs and withdrawn via the cannula inserted into the trachea. The cell numbers were counted using a hemacytometer. The BAL fluid was centrifuged at 1,300 rpm for 10 min and the supernatant was stored −80 °C. Differential cell counts were performed using slides prepared by cytocentrifugation and Diff-Quick staining. Approximately 500 cells were counted.
4. Measurements of IL-6 and TNF-α in the BAL fluids
The protein concentration in each sample was determined using a BCA kit (Pierce Biotechnology Inc., Rockford, IL., U.S.A.). IL-6 and TNF-α concentrations were measured with a quantitative sandwich enzyme-linked immunoassay kit (BD, San Diego, CA., U.S.A.). A 96-well microtiter plate was incubated overnight at 4 °C with anti-rat IL-6, TNF-α monoclonal antibody in coating buffer, washed with PBS containing 0.05% Tween 20 (Sigma, St. Louis, MO., U.S.A.) and blocked with 5% FBS in PBS for 1hr at room temperature. Subsequently 100 μl of BAL fluid was incubated for 2 hr at room temperature. Then secondary peroxidase labeled biotinylated anti-rat IL-6, TNF-α monoclonal antibody in 5% FBS in PBS diluents for 1 hr. Finally, the plates were treated with TMB substrate solution for 30 min and the reaction was stopped by addition TMB stop solution 50 μl per well. Optical density was measured at 450 nm in a microplate reader (SOFT max PRO software, Molecular Devices, Sunnyvale, CA., U.S.A.).
5. Lung Microscopic Studies
The right lower lobes were removed for histological analysis. Lung tissue were fixed in 4% paraformaldehyde solution and then embedded in paraffin. For histological examination, 4 μm section of embedded tissue were cut on a rotary microtome, placed on glass slides, deparaffinized, and stained sequentially with hematoxylin and eosin (H&E). The severity of lung inflammation was graded semi-quantitative as previously described17).
Hyperplasia of goblet cells within the bronchial epithelium was assessed by counting cells in periodic acid Schiff (PAS)-stained section. Slides were mounted with Canada balsam (Showa Chemical Co.Ltd., Tokyo, Japan). The ratio of PAS-positive cells/total cells in PAS stain slides was depicted. The air space was measured using Image-Pro plus 5.1 software (Media Cybernetics, Inc. Silver Spring, MD, USA).
6. Statistical analysis
Data are presented as the means ± S.E.M. Analysis of data was done using Graphpad Prism software (Graphad Software Inc., San Diego, CA., U.S.A.). The differences between study groups were determined by one-way ANOVA and Newman-Keuls Multiple Comparison test. P < 0.05 was considered to be statistically significant.
Results
2. Effect of Lonicerae Flos on the BAL cell profile
The effects of roflumilast and Lonicerae Flos on the number of BAL cells are shown in Figure 2. The increased number of neutrophils, lymphocytes, macrophages and total cells after exposure to smoking was observed. Cell count changes in BAL fluids were reflected in the histological morphology of the lung. Treatment with the Lonicerae Flos almost completely abrogated the inflammatory cell infiltration induced by cigarette exposure (Figure 2).
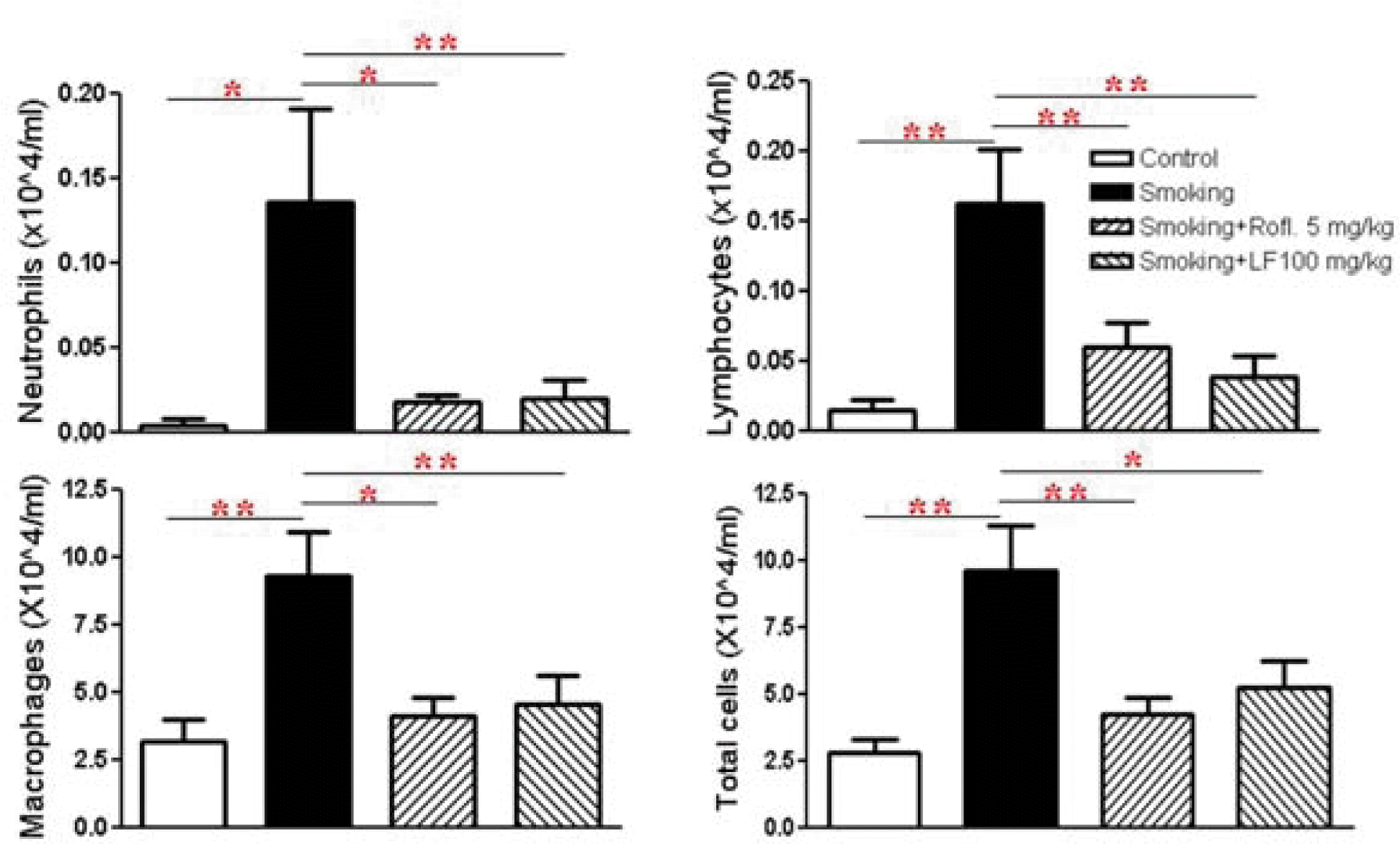
The Inflammatory cell profile in brochoalveolar lavage (BAL) fluid
BAL cells were then separated using a cytospin, after which they were stained with Diff-Quick stain. Differential cell counting was then performed using standard morphological criteria. ROFL: roflumilast, LF: Lonicerae Flos.
Data are presented as means ± SEM. * p < 0.05, ** p < 0.01, versus smoking: n = 5 – 6
3. The effect of Lonicerae Flos on cytokine in bronchoalveolar lavage (BAL) fluids of cigarette-induced mice
IL-6 and TNF-α are known to be pro-inflammatory cytokines that contribute to cigarette-induced lung inflammation. Therefore, the level of these cytokines in BAL fluid was measured. Data indicated that treatment with Lonicerae Flos significantly suppressed secretion of pro-inflammatory cytokines in cigarette-induced lung inflammation as effectively as roflumilast treatment.
4. Effect of Lonicerae Flos on cigarette-induced morphologic change
The results of histological examination of lung tissue paralleled the cell number in the BAL fluid. Marked influxes of inflammatory cells into the peribronchial layer and intraluminal areas were detected in the lung sections of cigarette- induced COPD mice. Additionally, Lonicerae Flos extract treatment led to a marked reduction in the infiltration of inflammatory cells within the lung in cigarette-induced COPD mice that was similar to the results observed in roflumilast-treated mice. These results demonstrate that treatment with Lonicerae Flos extract inhibits cigarette-induced inflammation in lung tissue in a fashion similar to roflumilast.
5. Lonicerae Flos extract decreased goblet cell hyperplasia and mucus production in a COPD mouse model
To evaluate the effect of Lonicerae Flos extract on goblet cell hyperplasia, lung tissues were stained using periodic acid-Schiff (PAS). The overproduction of goblet cell hyperplasia was detected in the bronchial airway epithelium of cigarette-induced COPD mice when compared to control mice. Additionally, all cigarette-induced COPD mice that received the Lonicerae Flos extract showed great reduction in goblet cells in the bronchial epithelium.
Discussion and Conclusion
Chronic obstructive pulmonary disease (COPD) is a very common public health concern worldwide, and the incidence of this disease is increasing globally18). COPD is cause by abnormal inflammatory response of the lung to the inhalation of noxious gases or suspended particles in the air. Cigarette smoke is the most common source of exposure causing COPD. Extended exposure to these irritants may lead to emphysema or obstructive bronchitis, the two hallmark conditions associated with COPD19). Cigarette smoking can drive the recruitment and activation of inflammatory immune cells, in particular neutrophils and macrophages, which induce the production of an array of inflammatory mediators including cytokines and chemokines in the airways that trigger mucus production, apoptosis of alveolar epithelial cells and matrix degradation, leading to chronic bronchitis and emphysema; all aspects of COPD pathology4,18,20–23). But how tobacco smoking causes COPD is not fully understood24).
Current conventional treatments of COPD are geared toward relieving symptoms, preventing recurrent exacerbations, preserving optimal lung function and enhancing the quality of life24). Although many drugs are used to treat COPD, adverse effects and side effects are associated with several classes of drugs, including steroids. In our cigarette smoke exposure models, the effects of extract from Lonicerae Flos on lung inflammation were similar to the effects of roflumilast, which was recently approved for treatment of severe COPD conditions, However, dose-limiting side effects such as vomiting, diarrhea, nausea, weight loss, emesis, and headache potentially limit the utility of roflumilast25–27). Therefore, there is a need for the development of efficacious and safe treatments for COPD28,29). Herbal medicines have long been used as an excellent source of anti-inflammatory agents with minimal side effects and are successfully employed in inflammation treatment, considered a harmless alternative to conventional medicine. We hypothesized that the anti-inflammatory effect of Lonicerae Flos may attenuate the progression of lung inflammation. A mouse model of cigarette-induced lung neutrophilia was used to mimic COPD-like lung disease30).
In this study, infiltration of inflammatory cells in BAL fluid and in the lung parenchyma was observed following exposure to cigarette smoke in mice. Concomitant with the influx of neutrophils, elevated levels of the pro-inflammatory cytokines, TNF-α and IL-6 were observed in the BAL fluid of cigarette-induced mice. The pathological changes observed in this study were similar to the clinical features of COPD patients31).
TNF-α and IL-6 were also decreased in the BAL fluid of roflumilast- and Lonicerae Flos-treated mice compared to cigarette-exposed mice. Studies have demonstrated that activation and infiltration of immune cells including macrophages and neutrophils play an important role in the expression of various pro-inflammatory cytokines and chemokines, including TNF-α, IL-1 β, CXCL-1, and MCP-112,19). TNF-α is produced by many different cell types. Macrophages, T-cells, B-lymphocytes, granulocytes, smooth muscles, eosinophils, chondrocytes, mast cells, glial cells and keratinocytes also produce TNF-α after simulation32). TNF-α also activates nuclear factor-κB (NF-κB), which increases IL-8 gene transcription, thereby inducing the release of IL-8 from the airway epithelium and neutrophils. IL-8, a CXC chemokine, is a neutrophil chemoattractant and activator31). Luteolin, which is a major flavonoid found in Lonicerae Flos, is known to inhibit an endotoxin-stimulated phosphorylation cascade and pro-inflammatory cytokine production in macrophages16). It is reported that luteolin suppresses inflammation-associated gene expression by blocking NF-κB in mouse alveolar macrophages33). In addition, the aqueous extract of Lonicerae Flos has been shown to exert potent inhibition of both NO production and TNF-α secretion in a dose-dependent manner in an activated macrophage-like cell line15). Therefore our data indicate that treatment with Lonicerae Flos may act on the influx of inflammatory cells and the levels of mediators aggravating the inflammatory response through modulating the NF-κB pathway.
In summary, the results of this study provide evidence that treatment with Lonicerae Flos exerts therapeutic effects against cigarette-induced lung inflammation in vivo. The remarkable effects exerted by Lonicerae Flos suggest that it has the potential for use in the treatment of COPD patients. However, further studies to elucidate the mechanisms by which the effects of Lonicerae Flos occur should be conducted to aid in the discovery of new therapeutic agents for the prevention of COPD.
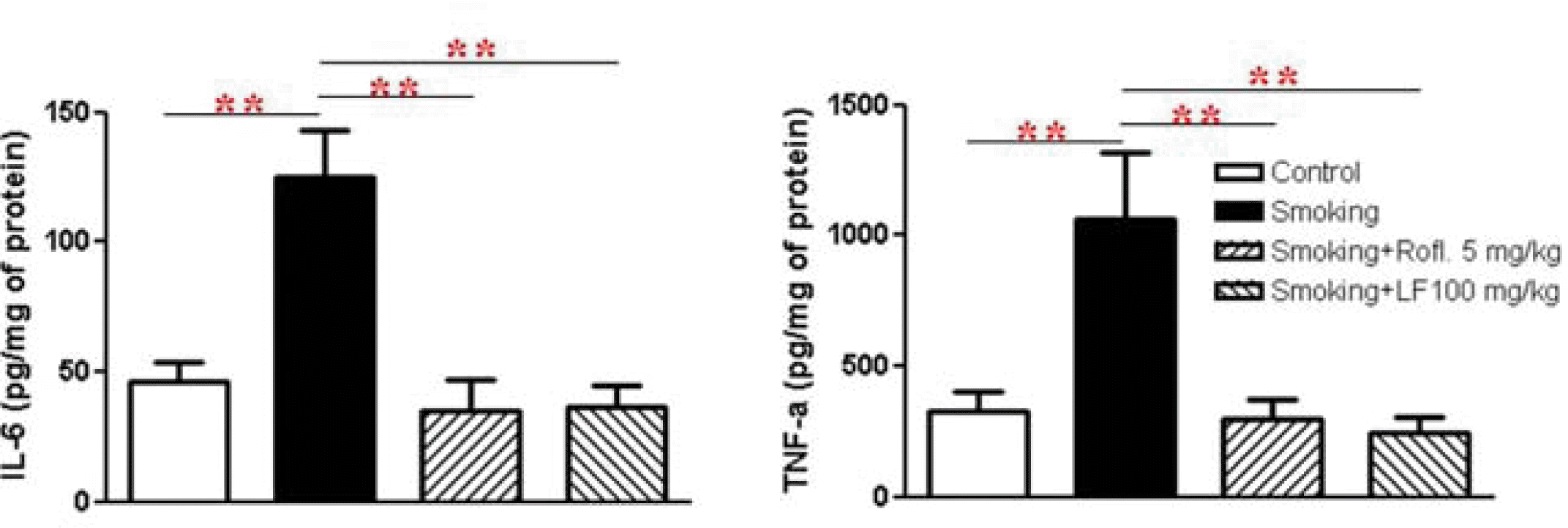
The effect of Lonicerae Flos on IL-6, TNF-α in BAL fluid.
BAL fluid was collected and the cells were eliminated. The cytokine levels were determined using a quantitative sandwich enzyme-linked immunoassay (ELISA) as described in methods and materials. Data are presented as means ± SEM. * p < 0.05, ** p < 0.01, versus smoking: n = 5 – 6
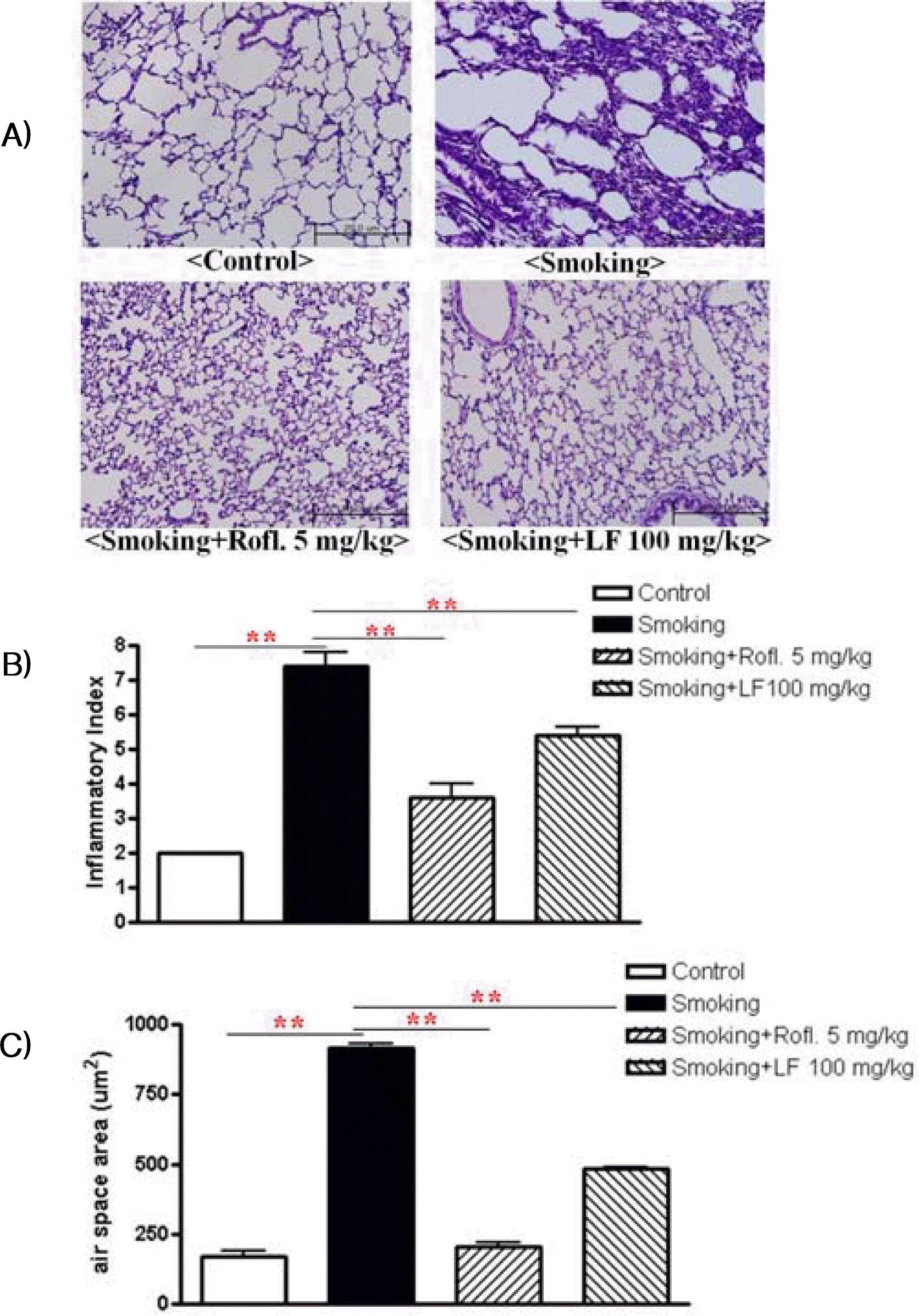
Effect of Lonicerae Flos on lung inflammation
Lung sections were stained with Hematoxylin & Eosin (H&E) for the analysis of tissue morphology. A) Non-stimulation mice: control, cigarette induced mice: smoking, cigarette induced mice treated with roflumilast: smoking+rofl., cigarette induced mice treated with Lonicerae Flos: smoking+LF. B) Semi quantitative analysis of lung inflammation were conducted based on the microscopic images obtained from A). C) Air space was measured using Image Pro-Plus 5.1 software as described in methods and materials. Data are presented as means ± SEM. * p < 0.05, ** p < 0.01, versus smoking: n = 5 – 6
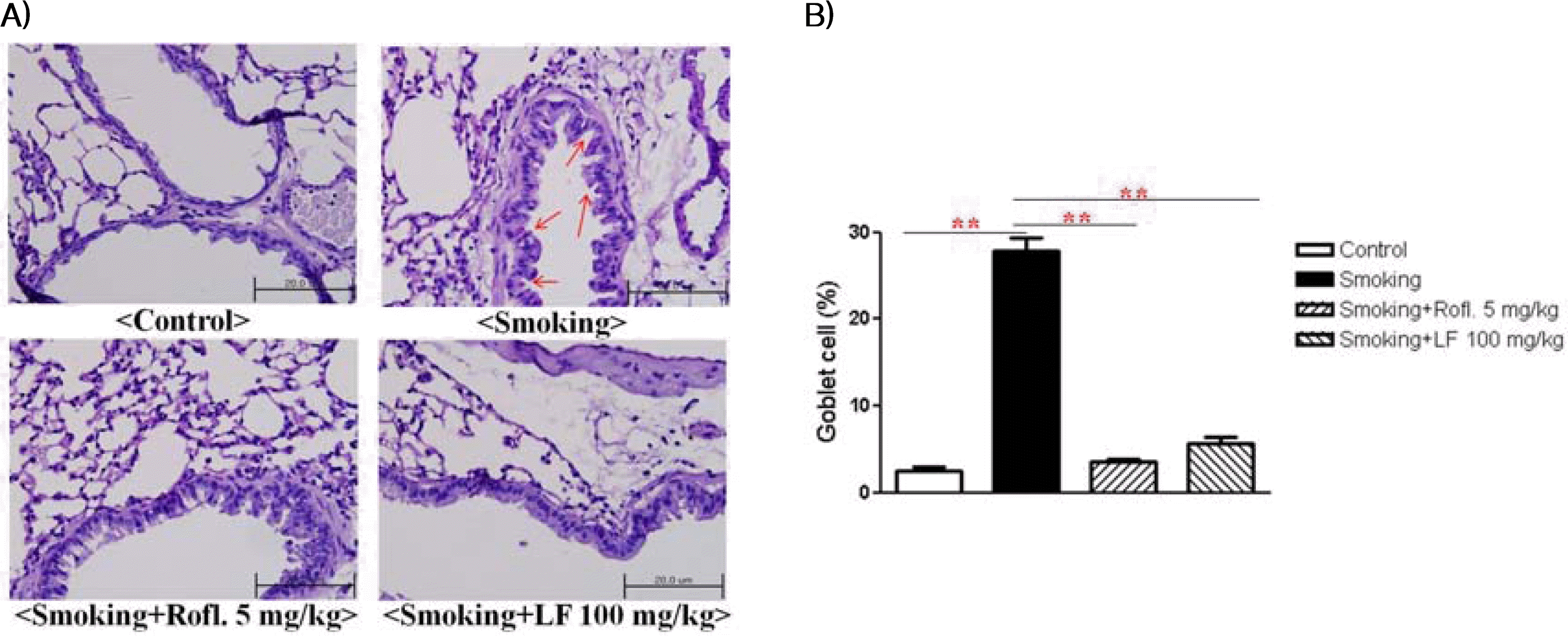
The effect of Lonicerae Flos on goblet cell hyperpiasia
A) Mice lung sections were stained with PAS. B) The percentage of PAS-positive cells was calculated. Non-stimulation mice: control, cigarette induced mice: smoking, cigarette induced mice treated with roflumilast: smoking+rofl., cigarette induced mice treated with Lonicerae Flos: smoking+LF.
Acknowledgements
This work was supported by a grant from Kyung Hee University in 2007 (KHU-20070627).