Introduction
Alzheimer’s disease is a neurodegenerative disease and the most common form of senile dementia. It is characterized by brain atrophy with histology, showing senile plaques, neurofibrillary tangles, and granulovacuolar degeneration1,2). Beta-amyloid (Aβ) is united to senile plaque in the extracellular environment, which causes neuronal apoptosis and activates neuroglia cells2). In the intracellular environment, tau protein produces neurofibrillary tangles forming filamentous shapes. It makes empty space surrounded by basophilic granules and granulovacuolar degeneration3,4).
So far, several possible causes of Alzheimer’s disease have been identified, including the reduction of acetylcholine, increase of acetylcholinesterase5), amyloid cascade hypothesis6), inflammatory response by neuroglia cell7), gene mutation of Presenilin-1 and Presenilin-28), and reactive oxygen species9). But the exact cause and pathogenesis is still unknown and many efforts have been carried out to clear it.
Currently, drugs for Alzheimer’s disease are directed at elevating the acetylcholine concentration of neurons by suppressing acetylcholinesterase. Acetylcholinesterase inhibitors like donepezil, rivastigmine, and galantamine are used for this purpose10,11). As understanding of the disease’s pathogenesis progresses, new treatments which relate with amyloid cascade hypothesis, are being developed12). The amyloid cascade hypothesis is a concept that focuses on the accumulation of beta amyloid as taking central role in Alzheimer’s disease, wherein its accumulation induces cell damage and cognitive impairment13).
Guibi-tang (GBT) is a herbal prescription used in traditional Korean medicine. It consists of 12 different herbs14). Under admission of Korean Food and Drug Administration (KFDA), it has been used as a remedy for anemia and insomnia. The major ingredients of Guibi-tang are nodakenin, decursin, ginsenoside Rg1, liquiritin, and glycyrrhizin15).
In an in vitro experimental study, it has been reported that Guibi-tang reduces apoptosis in rat microglia oxidative damage model and astrocyte glutamate damage model16,17). Similarly, in PC12 cells treated with 6-Hydroxydopamine (6-OHDG), Guibi-tang dose-dependently reduces neuronal apoptosis18). In a recent animal study, Guibi-tang has induced cell proliferation and promoted learning and memory in the hippocampus dentate gyrus (DG)19). Improved cognitive function in Alzheimer’s disease patients has been shown in a clinical trial20). This study attempted to examine the impact of Guibi-tang in Alzheimer’s disease.
This study confirmed the pathological state of Aβ injected mouse by cresyl violet stain and Aβ accumulation in the hippocampus CA1 and DG region. Neuronal apoptosis has been confirmed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) stain. This study examined the effect of Guibi-tang administration on neuronal apoptosis in mice induced by intraventricular Aβ injection and the restoration of cognitive impairment caused by the neuronal damage using the Morris water maze.
Materials and Methods
1. Preparation of GBT
The twelve crude herbs of GBT were purchased from Puremind Medicinal Herbs (Youngchen, Korea). A decoction of GBT was prepared in our laboratory from a mixture of chopped crude herbs (Table 1). GBT was extracted in distilled water at 100°C for 3h. The solution was evaporated to dryness and freeze-dried (yield: 20.0%). The extracted GBT powder was stored at 4°C.
2. Animals
Male C57BL/6 mice (25–28g, Nara Biotechnology, Korea) were used for this study. All animal protocols were approved by the Ethics Committee for the Care and Use of Laboratory Animals at Kyung Hee University. The animals were housed in plastic cages at constant temperature (22±2°C) and humidity (55± 10%) with 12 h-12 h light-dark conditions. The animals were allowed free access to food and water before the experiment.
3. Aβ (1–42)-induced AD mouse Model
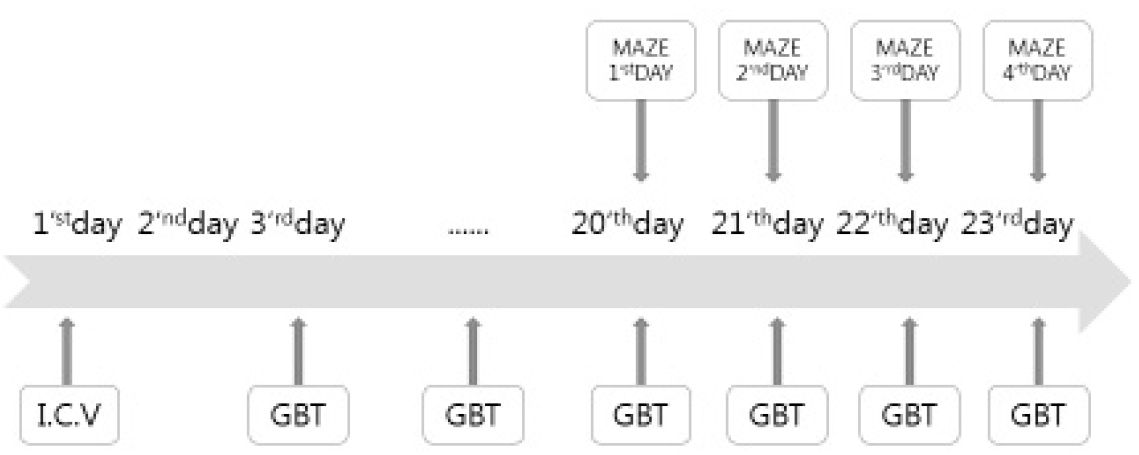
The Aβ (1–42) (#A9810, Sigma, St. Louis, MO, USA) was dissolved in sterile saline to a concentration of 0.66 mg/ml and incubated at 37°C for 4 days to allow for fibril formation. The mice were anesthetized with Zoletil (30 mg/kg, i.p.) and placed on a stereotaxic instrument (USA). The scalp of each mouse was incised, and the skull was adjusted to place the bregma and lambda on the same horizontal plane. A small bone hole was drilled through the skull. Aβ (1–42) (3ul=2ug) or the vehicle (3 μl) was injected into a lateral ventricle (−0.5 mm anteroposterior, +1.5 mm medial-lateral, −2.5 mm dorsal-ventral from the dura, in relation to the bregma) at a rate of 0.5 μl/min using a 10ul Hamilton syringe fitted with a 26-gauge stainless steel needle. The hole was blocked with bone wax and the scalp was then closed with suture. Sham group mice received the same surgical procedure with injection of an identical volume vehicle (sterile saline).The mice were allowed to recover from surgery for 2 days. Saline and Guibi-tang (500 mg/kg) were administered intragastrically once daily for 21 days beginning from two days after the Aβ (1–42) injection.
4. Experimental groups
The mice were randomly divided into three groups. The Aβ and GBT groups were injected intracerebroventricularly with a Aβ (1–42)(3ul=2ug). The sham group received the same surgical procedures and was injected with an identical volume of vehicle (sterile saline). The GBT group received Guibi-tang (500 mg/kg, dissolved in normal saline, orally), once a day for 21 days from two days after the Aβ (1–42) injection. The Aβ and sham group received vehicle (normal saline) orally. A total of 36 mice were used.
5. Tissue Processing
The mice were anesthetized and transcardially perfusion-fixed with 4% formaldehyde in 0.1M sodium phosphate buffer (pH 7.4). Brains were removed quickly and postfixed with the same fixation solution overnight at 4°C. Fifty μm thick coronal sections of brain tissue were made using a freezing microtome (Leica, 2800N, Germany).
6. Morris water maze test
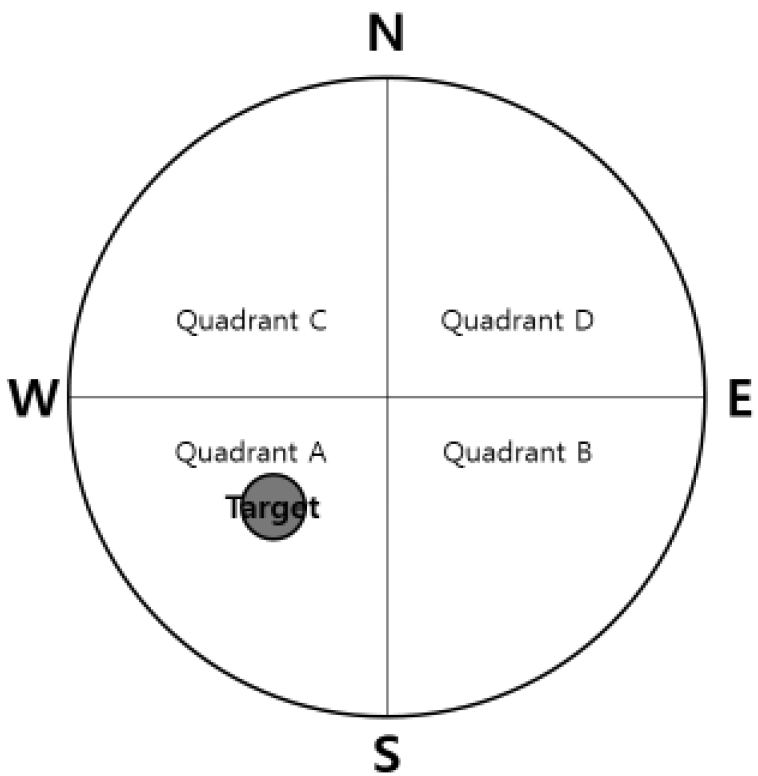
The Morris water maze test was performed for 4 days. The acquisition training was performed for 3 days and the retention test on the 4th day. The apparatus consisted of a circular water pool 100 cm in diameter and 40 cm in height. It was filled with 23 ± 1°C water with a depth of 28 cm and covered a black platform (10 cm in diameter). The platform was submerged approximately 0.5 cm below the surface of the water. The pool was divided into four equal quadrants: northeast (NE), northwest (NW), southeast (SE), and southwest (SW). The platform was located in the center of the southwest quadrant. During the first 3 days acquisition test, mice were given 8 trials per day to find the hidden platform. Each mouse (12 mice per group) was gently placed into the water facing the wall in the direction of north (N), east (E), south (S), and west (W) in two series of order. The mouse was allowed to swim until they reached the hidden platform (maximum swim time was 60 seconds). The escape latency to reach the platform was recorded and they were allowed to remain on the platform for 10 seconds before being removed. Any mice which failed to find the platform within 60 seconds were guided to the hidden platform and then placed on the platform for 10 seconds for reinforcement before being removed. One trial of the retention test without the platform was performed on the 4th day to assess the memory of the correct platform location. The mice were placed into the pool and swam freely for 60 seconds. The swimming paths were recorded by a video camera linked to a computer-based image analyzer (SMART 2.5 video-tracking system, Panlab, Spain). The number of target headings and the swimming time in each zone were analyzed with a grid design of 4 zones (Fig. 2). This grid design, constructed with a computer-based image analyzer, was superimposed over the maze and viewed on a monitor. The mice were sacrificed after the retention test trial.
7. Cresyl violet staining, Congo red staining, and TUNEL labeling
Neuronal damage and apoptosis in the CA1 of the hippocampus were observed using cresyl violet staining, Congo red staining, and terminal transferase dUTP nick-end labeling (TUNEL) assay. The hippocampal tissues analyzed came from the mice which performed the Morris water maze test. Cresyl violet staining was used; hippocampus tissues were immersed in 0.5% cresyl violet solution for 3 minutes. Congo red staining was used; hippocampus tissues were immersed in 0.3% Congo red stock solution for 20 minutes. The hippocampus TUNEL labeling was carried out using In Situ Cell Death Detection kit, POD (#11684817910, Roche, Germany) following the manufacturer’s protocol.
8. Image analysis
Relative optical densities of Congo red, counting of the CA1 and DG neurons stained with cresyl violet and TUNEL-labeled cells were analyzed using ImageJ software (Ver. 1.44p, NIH, USA). The relative optical densities were measured in the CA1 and DG by the mean gray value on an inverted black-white binary image and were normalized to the value of the normal group. The number of CA1 neurons were counted in the 500 μm length of the pyramidal cell layer of CA1. TUNEL-labeled cells were measured from a 104 μm area of the CA1 and DG region. Data were normalized with the same area. The mean values from the four sections analyzed in each mouse were used for statistical analysis.
9. Statistical analysis
All data in this study are presented as means ± standard errors and were evaluated using Student’s t-test. A probability value of less than 0.05 was used to indicate a significant difference. Differences between groups in the acquisition test were evaluated using one-way ANOVA.
Results
1. Acquisition trials analysis
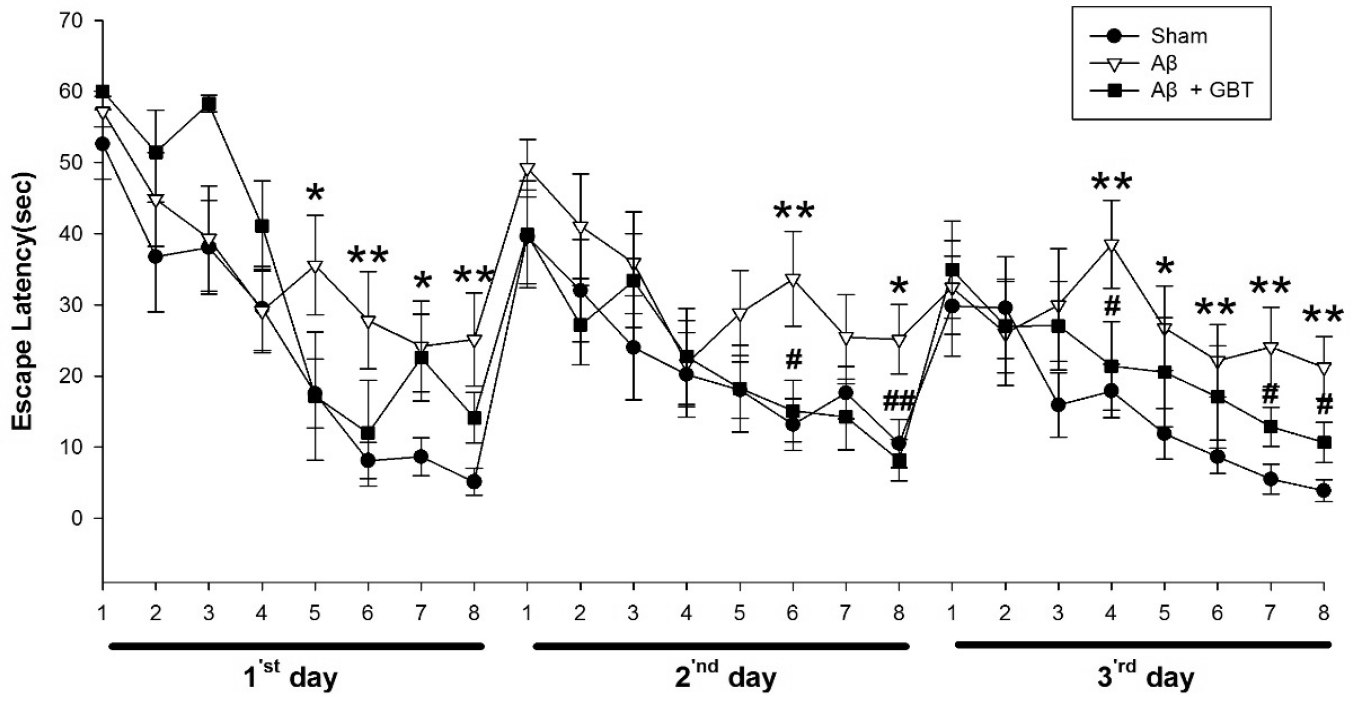
Fig. 3 demonstrates escape latencies in the training period for three days. The Aβ group had significantly longer acquisition time than the sham group at the 5th, 6th, 7th, and 8th trials (p<0.05, p<0.01, p<0.05, p<0.01, respectively) on the 1st day, 6th and 8th trials (p<0.01, p<0.05, respectively) on the 2nd day and 4th, 5th, 6th, 7th and 8th trials (p<0.01, p<0.05, p<0.01, p<0.01, p<0.01, respectively) on the 3rd day. The GBT group showed significantly less acquisition time at the 6th and 8th trials (p<0.05, p<0.01) on the 2nd day and 4th, 7th, and 8th trials (p<0.05, respectively) on the 3rd day.
There were many trials that had shorter escape latency on the 3rd day compared to the 1st and 2nd days. On days 1, 2, and 3, the shortened escape latencies occurred towards the latter half of the 8 trials.
2. Retention trials analysis
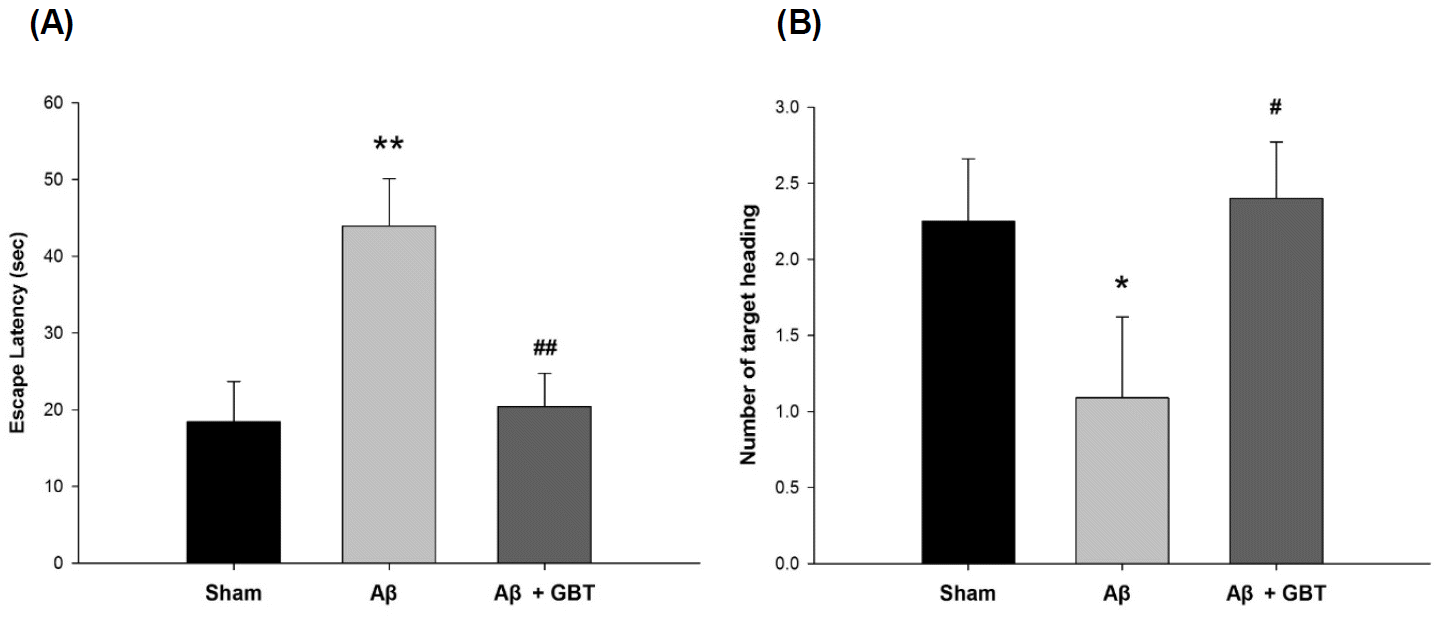
Upon completion of the acquisition trials, the escape latency and number of target headings were examined without the platform for 60 seconds on the 4th day. The escape latency of the Aβ group was 44.0±6.2 s and was significantly longer than in the sham group, which was 18.4±5.3 s (p<0.01). The escape latency of the GBT group was 20.4±4.4, a significantly shorter time compared to that of the Aβ group (p<0.01). The number of target headings for the Aβ group was 1.1±0.5. This was significantly smaller compared to the sham group’s 2.3±0.4 (p<0.05). The number of target headings for the GBT group was 2.4±0.4. This was significantly larger compared to that of the Aβ group (p<0.05).
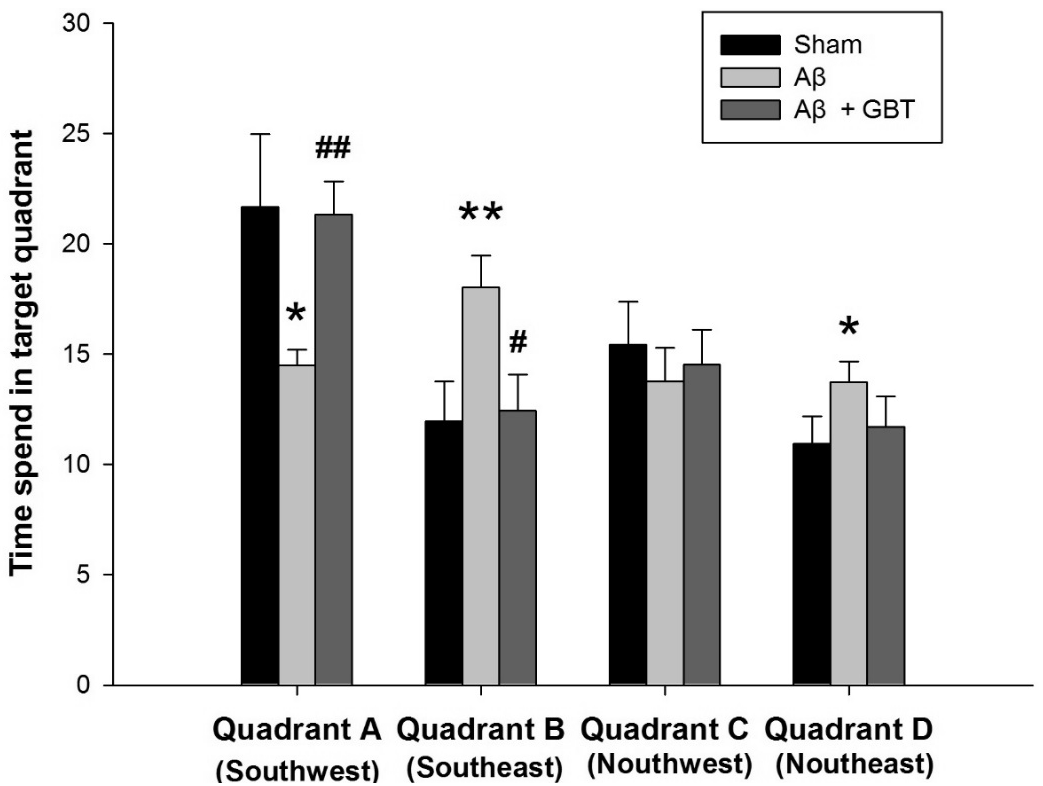
3. Swimming time spent in discrete quadrants
After completion of the acquisition trials, the tracking path without the platform was examined for 60 seconds on the 4th day. Swimming time in discrete quadrants for the sham group were: 21.7± 3.3 in Quadrant A (where the platform was placed previously), 12.0±1.8 in Quadrant B, 15.4±2.0 in Quadrant C, and 11.0±1.2 in Quadrant D. Swimming time in discrete quadrants for the Aβ group were: 14.5±0.7 in Quadrant A (where the platform was placed previously), 18.0±1.5 in Quadrant B, 13.8±1.5 in Quadrant C, and 13.7±0.9 in Quadrant D. The Aβ group swam for a significantly shorter time in Quadrant A compared to the sham group (p<0.05). The Aβ group swam in Quadrants B and D for a significantly longer time compared to the sham group (p<0.01, p<0.05, respectively). Swimming time in discrete quadrants for the GBT group were: 21.3±1.5 in Quadrant A (where the platform was placed previously), 12.4±1.7 in Quadrant B, 14.5±1.6 in Quadrant C, and 11.7±1.4 in Quadrant D. The GBT group showed significantly more time in Quadrant A (p<0.01) and significantly less time in Quadrant B compared to the Aβ group (p<0.05).
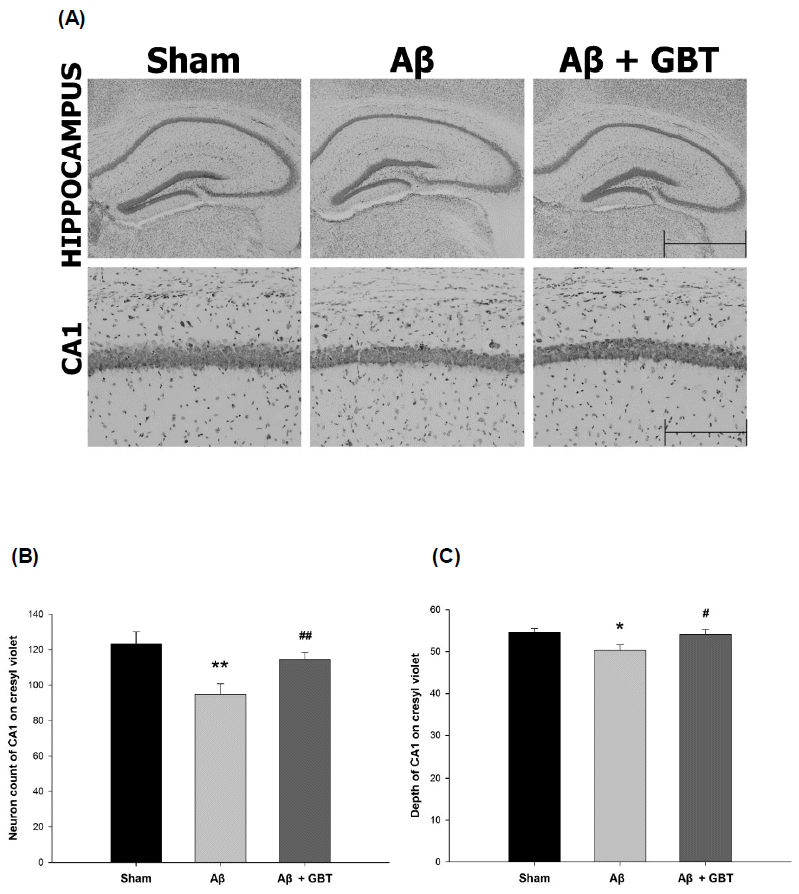
Guibi-tang attenuated neuronal cell loss in the hippocampus of Aβ (1–42)-injected mice
Neuronal cell loss was assessed by counting the number and observing the morphology of neuron cells in the hippocampus using cresyl violet staining. The Aβ group showed short and thin neuron cell layers in the hippocampus CA1 region compared to the sham group. This change meant that the number of neurons decreased. In contrast, the GBT group exhibited thick and deep neuron cell layers.
The number and depth of neuron cell layers was inspected in certain hippocampus CA1 regions. The number of CA1 neurons was 123.2±7.1 in the sham group and 94.8±6.0 in the Aβ group. The Aβ group showed significantly fewer CA1 neurons compared to the sham group (p<0.01) while the GBT group showed significantly more CA1 neurons (114.4±4.2) compared to the Aβ group (p<0.01). The depth of the CA1 region in the sham group was 54.6±0.9 and was 50.3 ± 1.3 in the Aβ group. The Aβ group showed significantly shallower CA1 compared to the sham group. The GBT group showed significantly deeper CA1 (54.1±1.1) compared to the Aβ group.
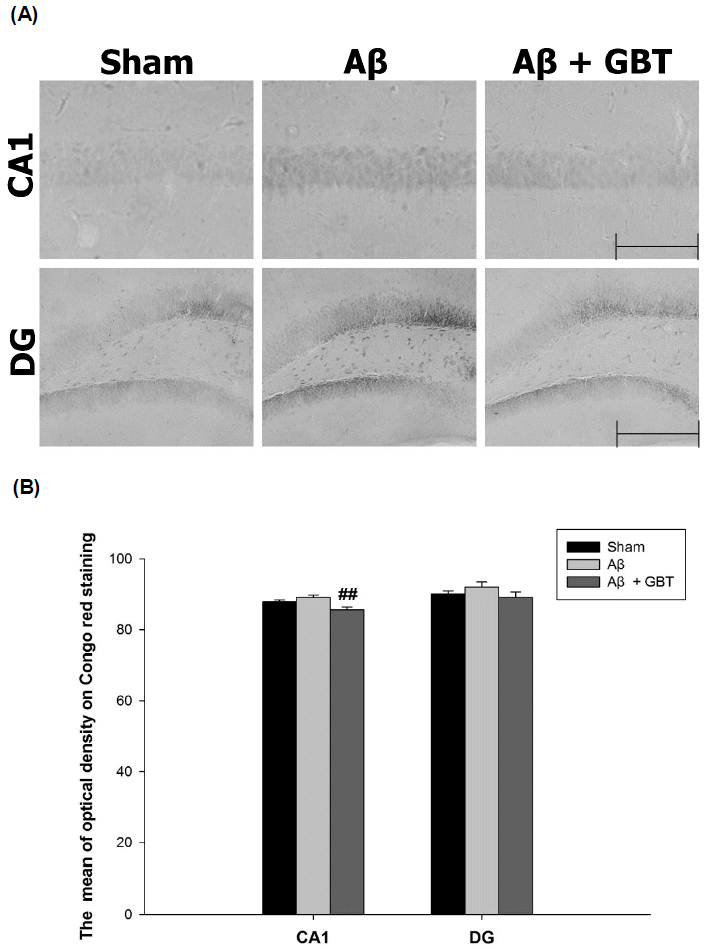
Guibi-tang attenuated Aβ accumulation in the hippocampus of Aβ (1–42)-injected mice
The optical density of Congo red staining of Aβ accumulation was observed in certain hippocampus CA1 and DG regions. The optical density of Congo red staining in the CA1 region for the sham and Aβ group were 87.8±0.5 and 89.1±0.7, respectively. The Aβ group’s value increased compared to the sham group’s but not significantly. The optical density of Congo red staining in the CA1 region for the GBT group was 85.7±0.7, which was significantly lower compared to the Aβ group (p<0.01). The optical density of Congo red staining in the DG region for the Aβ group was higher compared to the sham group but not significantly. There was also no significant difference in the optical density of Congo red staining in the DG region between the GBT and Aβ groups.
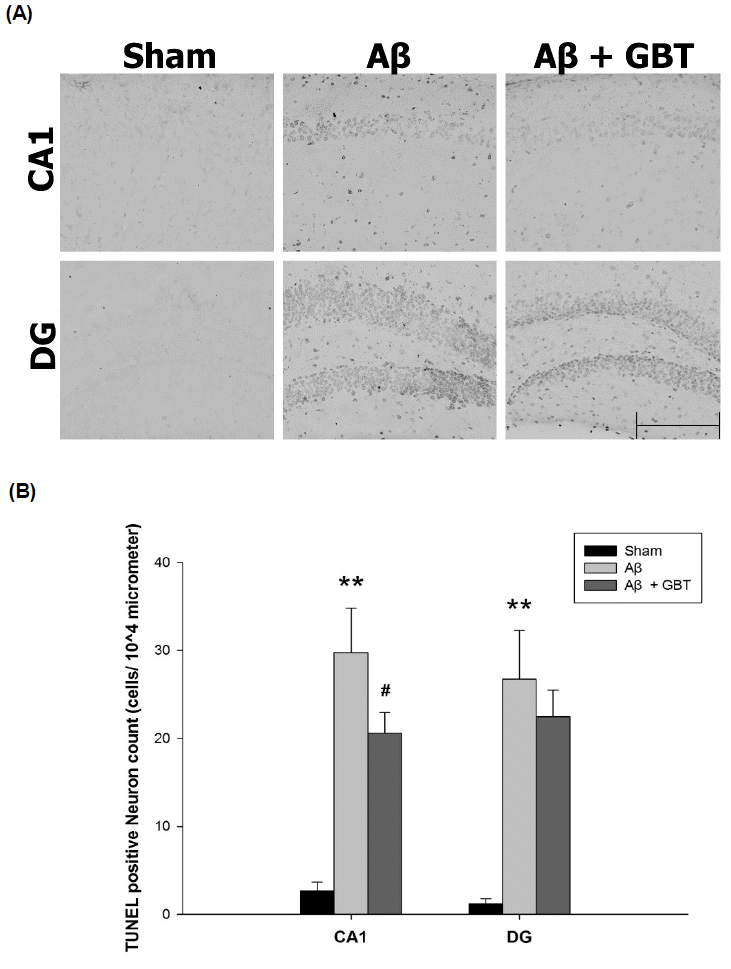
Guibi-tang attenuated neuronal apoptosis in the hippocampus of Aβ (1–42)-injected mice
Neuronal apoptosis was examined using TUNEL assay that stains DNA fragments as a result of cell death. TUNEL-labeled cells were rarely seen in the CA1 and DG regions of the sham group. Many TUNEL labeled cells were seen in the Aβ and GBT groups. The number of TUNEL-labeled cells were measured from a 104 μm area of the CA1 and DG region. The Aβ group showed significantly increased TUNEL-labeled cells in the CA1 region compared to the sham group (29.8 ± 5.0 vs. 2.7 ± 1.0 cells/104 μm, p<0.01). The GBT group showed significantly fewer TUNEL-labeled cells in the CA1 region compared to the Aβ group (20.6 ± 2.4 vs 29.8 ± 5.0 cells/104 μm, p<0.05). The Aβ group showed significantly more TUNEL-labeled cells in the DG region compared to the sham group (26.8 ± 5.5 vs. 1.2 ± 0.6 cells/104 μm, p<0.01). The number of TUNEL-labeled cells in the DG region was similar between the GBT and Aβ groups (22.5 ± 3.0 vs 26.8 ± 5.5 cells/104 μm).
Discussion
Available evidence indicates that Guibi-tang is effective in the management of learning, memory, and cognitive impairment19). We analyzed the effects of Guibi-tang administration as a possible Alzheimer’s disease treatment. Using an Alzheimer’s disease animal model, we tested Guibi-tang’s effect on the amyloid cascade hypothesis, neuronal apoptosis, and cognitive impairments as well as Guibi-tang’s potential neuron protective effect.
The Morris water maze test, which confirms space reference memory and space working memory, was conducted to determine improvement of cognition. The GBT group showed improvement in advanced learning memory and cognition. Significant results during the training trials were mostly concentrated towards the tail end of the trials (14th, 16th, 20th, 23th, and 24th of 24 innings). The favorable effect of Guibi-tang was significant during the 2nd and 3rd day out of the three-day training period. Another Guibi-tang study showed similar favorable results in the rear of the training trials (11th, 12th and 13th of 16 inning)21). Concentration of significant results towards the rear of trial series suggests that Guibi-tang had excellent effect in repeated learning memory. Learning and memory are major problems in Alzheimer’s disease, which impedes the patients from having a normal daily life. An improvement in learning memory among patients with Alzheimer’s disease as a result of Guibi-tang can be valuable. Retention tests showed that the GBT group had 90.2 % recovery compared to the sham group on escape latency. In particular, the 98.2% recovery on time spent in the target quadrant showed that Guibi-tang had a greater effect than ferulic acid, which effected an 86% recovery in another study that employed a similar experimental design22). A study that induced dementia by scopolamine showed that GBT treatment shortened the escape latency more than tacrin treatment23), wherein tacrin is already a known remedy for dementia. From these experimental results, it appears that Guibi-tang can effect an improvement of memory deficit and cognitive impairment in diverse conditions.
The Aβ (1–42) induced brain tissue damage, confirmed by using cresyl violet staining, was attenuated by Guibi-tang. Abnormal accumulation of Aβ is a major factor in Alzheimer’s disease. It is closely related with memory and cognition impairment, wherein Aβ forms senile plaques outside the neurons. Alzheimer’s disease is also associated with a reduction in neurons that are related with memory and cognition in the cerebral cortex and hippocampus24, 25). A study that utilized Aβ injection confirmed that neurons were reduced in the hippocampus including CA1, CA2, and DG after 6 weeks26). According to the cresyl violet staining we conducted, there was significant decrease in the number of neurons and layer depth in the CA1 region of the hippocampus 3 weeks after Aβ (1–42) was injected into the lateral ventricle of the brain. The GBT-treated group recovered 92.8% of the neuron cell numbers and 99.1% of the neuron cell layer depth compared to the sham group. This showed that Aβ-induced brain tissue damage can be restored by Guibi-tang. We hypothesize that Guibi-tang may have a neuron protective effect by disrupting neuronal apoptosis.
Aβ (1–42) accumulation in brain tissue was confirmed by using Congo red staining. Congo red staining is most commonly used to observe formation and cohesion of Aβ fibril27). Congo red stains a cross β-pleated sheet present on the Aβ fibril. Congo red does not stain the monomer and dimer states of Aβ, allowing Congo red to confirm pathological condition of Aβ accumulation28). Aβ can accumulate in various brain regions after it is injected into the lateral ventricle of the brain. A study was able to immediately confirm Aβ accumulation by injecting fluorescent labeled Aβ (25–35) into the lateral ventricle, the 3rd and 4th ventricles, but accumulation of Aβ in the hippocampus was detected three days after injection29). At least three days is needed for Aβ to accumulate in the hippocampus after it is injected into the lateral ventricle. Our experiment was done over a 3-week period after the Aβ injection.
The Congo red staining showed the Aβ group had more Aβ accumulation in the hippocampus CA1 and DG region than the sham group, but their optical densities did not differ significantly. Methods that can better quantitate Aβ accumulation like western blotting or ELISA are needed for further study. The GBT group had reduced Aβ accumulation compared to the Aβ group with the accumulation in the hippocampus CA1 region, being suppressed significantly. CA1 has more neuronal apoptosis than other hippocampus regions in Alzheimer’s disease patients30). It can be relevant to Alzheimer’s disease that Guibi-tang reduced Aβ accumulation in CA1.
We confirmed neuronal apoptosis by TUNEL labeled neuron. Neurons have normal morphology during apoptosis. Cohering a chromatin in the nucleus, the neuron starts to atrophy and fragment31). DNA fragments are formed by the cutting of the nucleosome. TUNEL staining detects this fragmentation32). The Aβ group had significantly more TUNEL-labeled cells in the hippocampus CA1 and DG regions compared to the sham group. The GBT group had a reduced number of TUNEL-labeled cells with significant reduction in the CA1 region.
Guibi-tang reduced Aβ accumulation in the CA1 region and reduced neuronal apoptosis induced by Aβ. Guibi-tang’s cognition improvement effect appears to be due to the reduction of neuronal apoptosis. Considering the irreversible nature of Alzheimer’s disease, the above result is meaningful. Our study is the first to confirm that Guibi-tang reduced tissue damage through suppression of neuronal apoptosis in an Alzheimer’s animal model, in vivo study. We did not compare Guibi-tang and other chemical drugs because Guibi-tang is a crude water extract and it is difficult to compare dosage with chemical drugs. Further study about comparative efficacy with chemical drugs is needed.
Guibi-tang improved Aβ-induced memory impairment in mice, and attenuated Aβ accumulation and neuronal apoptosis in the hippocampus CA1 region. As a result, this can explain improvement of cognitive impairment.