Introduction
Hair loss is dramatically growing trend to modern civilized people, and can be occurred by hereditary causes, aging, diseases, malnutrition, psychological stress, hormonal imbalances, etc1). Clinically, hair loss is considered a non-inflammatory disease. However, recent studies show that the oxidative stress and inflammation also play a pathogenic role in hair loss. The perifollicular inflammation is observed in the scalp of hair loss patients2,3).
The hair growth is a dynamic and processes in repeated growth cycles divided into anagen (growth stage), catagen (regression stage), and telogen (resting stage). The anagen stage enlarge the hair follicles and promote the differentiation and proliferation of hair matrix keratinocytes. The catagen stage induce apotosis-deriven regression of hair follicles and the telogen stage keeps hair rest. Dysregulation of growth cycle is closely associated with hair loss and anagen-catagen transformation is important to understand the hair growth disorder4).
Minoxidil, the most popular drug for hair loss treatment, accelerates hair growth by extending anagen stage. However, minoxidil has a transient and unpredictable efficacies and may cause adverse effects such as local irritation and dermatitis5).
Rumex japonicas Houttuyn (RJH) is a traditional Chinese medicine with multiple beneficial effects to treat skin disease such as itch, psoriasis, abscess, dermatitis6). Recent study has reported RJH contains anti-bacterial, anti-malarial, anti-oxidant, and anti-inflammatory activity in skin disease7–11). It was also showed that RJH has hair growth promotion effects in mouse models and proliferate effects in keratinocytes and dermal papilla cells12).
In this study, we further investigated and the effects of H2O2-induced oxidative toxicity and Reactive oxygen species(ROS) generation in addition to the hair growth promotion effect of Rumex japonicas Houttuyn ethanol extract (RJHEE). For this, we investigated the effect of RJHEE on C57BL/6 mice and cultured human keratinocyte (HaCaT) cells.
Materials and Methods
1. Materials
C57BL/6N mice (SAMTAKO BIO KOREA Co., LTD., Osan, Kyungkido, Korea) and human keratinocyte (HaCaT) cells (American Type Culture Collection, Manassas, VA, USA) bikiro cream (Tai Guk Pharm. co. LTD, Hwaseong, Korea), and minoxidil (MXD, Hyundae Pharm. Co., LTD, Cheonan, Korea) were purchased from indicated sources. Antibodies and reagents used in this study were as follows: anti-Wnt3α, anti-phospho GSK3β(Ser9), anti-β-catenin, anti-ERK, anti-phospho ERK, Bax, Bcl-xl, Bcl-2, and β-actin (Cell Signaling Technology, Danvers, MA, USA), goat anti-mouse IgG (HRP)-conjugated secondary antibody (Enzo Life Sciences, Inc., Farmingdale, NY 11735 USA), hematoxylin (BBC Biochemical, Mount Vernon, Washington, USA), eosin (Shimakyu’s Pure Chemicals, Osaka, Japan), Fetal bovine serum (FBS), Dulbecco’s Modified Eagle’s Medium (DMEM), phosphate-buffered saline (PBS) and other tissue culture reagents (Gibco BRL Co. Carlsbad, CA, USA). Propyleneglycol,3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Dimethyl sulfoxide (DMSO) and reagents without indication were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
2. Preparation of Rumex japonicas Houttuyn ethanol extract(RJHEE)
The dried roots of RJH, were purchased from Omniherb Co., Ltd. (Daegu, Korea) and the roots were washed 3 times with distilled water to eliminate impurities. A total 50 g of the dried roots were extracted with 500 ml of 25% ethyl alcohol for 48 hours at 37°C. The 25% Rumex japonicas Houttuyn ethanol extracts (RJHEE) were filtered and evaporated using a rotary evaporator (Tokyo Rikakikai Co., Ltd, Tokyo, Japan) under a reduced pressure. The extract was subsequently lyophilized, and the extract yield was ~21%. A voucher specimen (DKMP 201606 RJHEE) was deposited at Korean Medical Physiology Laboratory, Dongeui University. The extracted powder was stored at 20°C until further use.
3. Animals and treatment
C57BL/6 male mice were purchased from SamtacoBioKorea Co., LTD (Osan, Korea) and were 6 weeks old at the initiation of the study. Mice were kept in a temperature controlled room (at 23 ± 1°C) with relative humidity of 50 ± 10% and a 12 h light/dark cycle. The mice were housed in polystyrene cages at Dongeui University and given standard rodent chow and water ad libitum. The animals in this study were cared for according to the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, D.C., U.S.A.). The experimental protocol was approved by the Institutional Animal Research Committee of Dongeui University on Animal Care and Use (approval number: DEU-R2017-027), and all efforts were made to minimize animal suffering and reduce the number of animals used for the experiments.
It has been reported that the depilation-induced hair cycle has a well-regulated course. The hair follicles get into the final stage of the anagen around day 9 after depilation, the catagen around day 17 after depilation, and the telogen around day 20 after depilation13). The 6-week-old male C57BL/6N mice were shaved to trigger a new anagen phase and used to observe skin colorimetric and early pigmentation changes14).
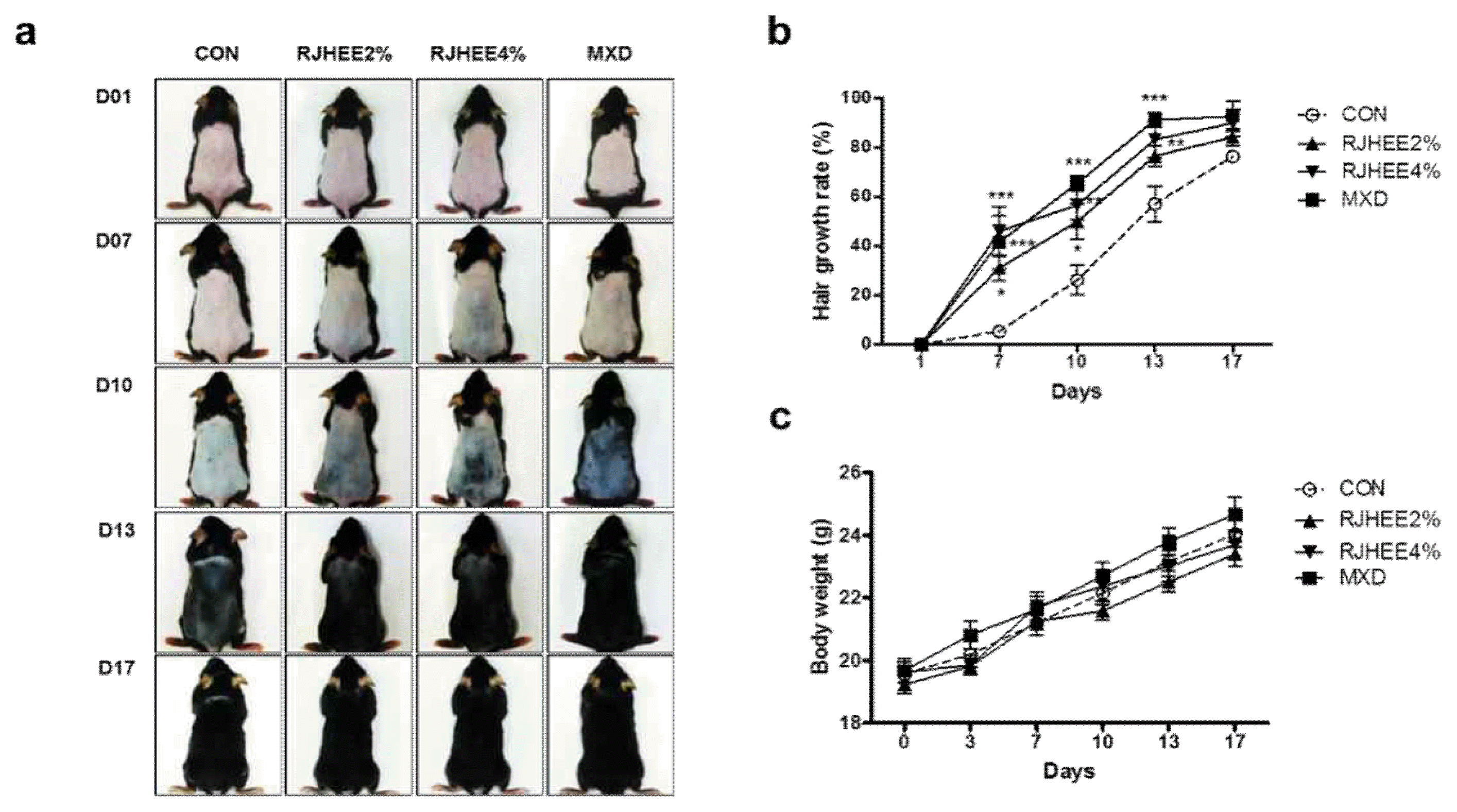
From the following day (day 1), C57BL/6 mice were randomized into four groups of 8 animals each: the vehicle mixture(ethanol:propyleneglycol, 3:7%v/v)-treated group (CON), 2% RJHEE-treated group (RJHEE2%), 4% RJHEE-treated group (RJHEE4%), and 2% minoxidil-treated group (MXD). RJHEE and minoxidil were dissolved in the same vehicle mixture described. Experimental groups topically applied with 200 μl of the vehicle mixture, 2% and 4% RJHEE, and 2% minoxidil to the back skin of mice daily for 17 days, respectively. Changes in hair growth were recorded by photographs using a digital camera and Folliscope (Lead M, Seoul, Korea) on days 1, 7, 10, 13, and 17. After the completion of the experiments, the mice were sacrificed by diethyl ether inhalation, and the backskin tissues were removed and stored at −70 °C until analysis.
4. Determination of hair growth-promoting activity
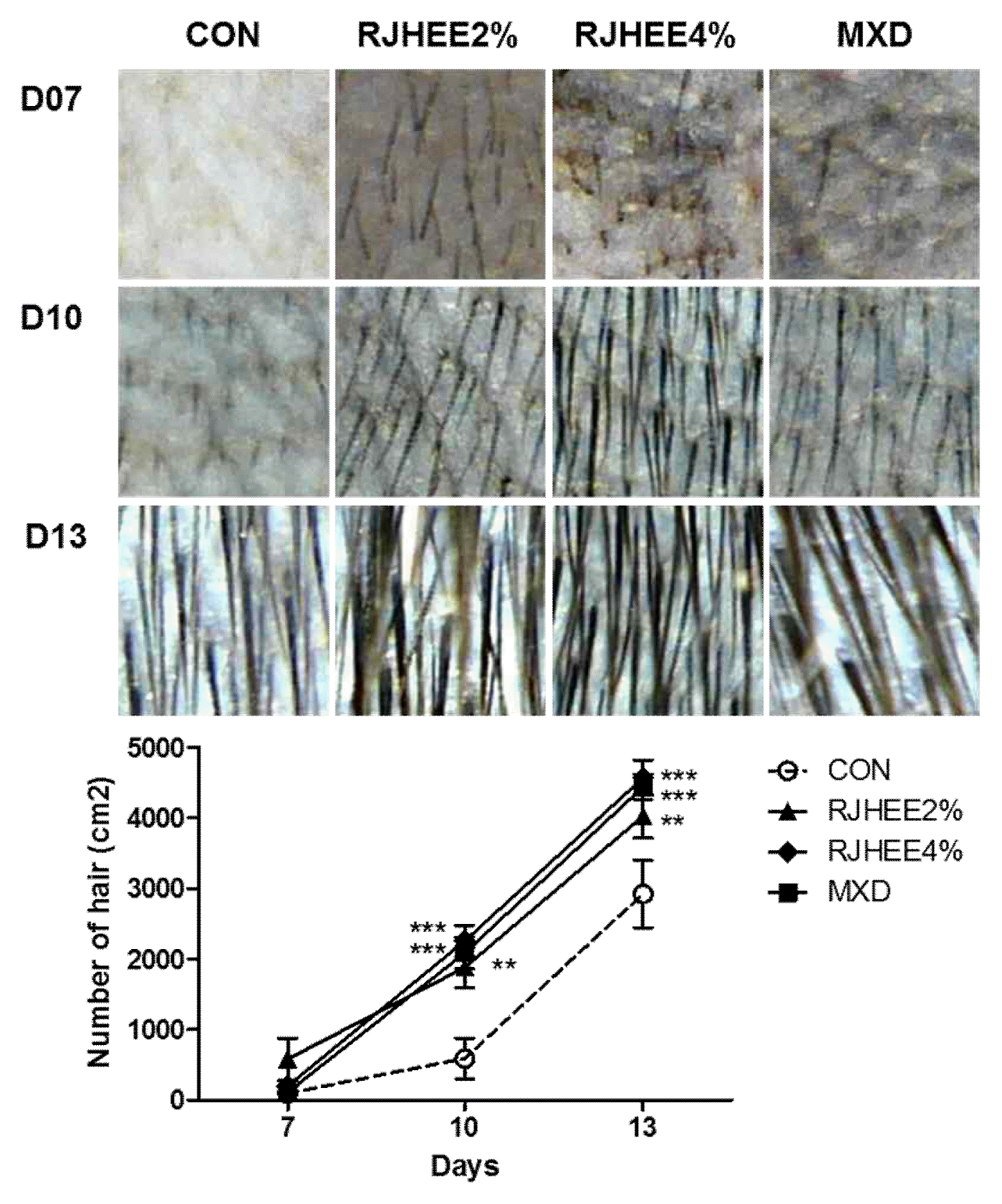
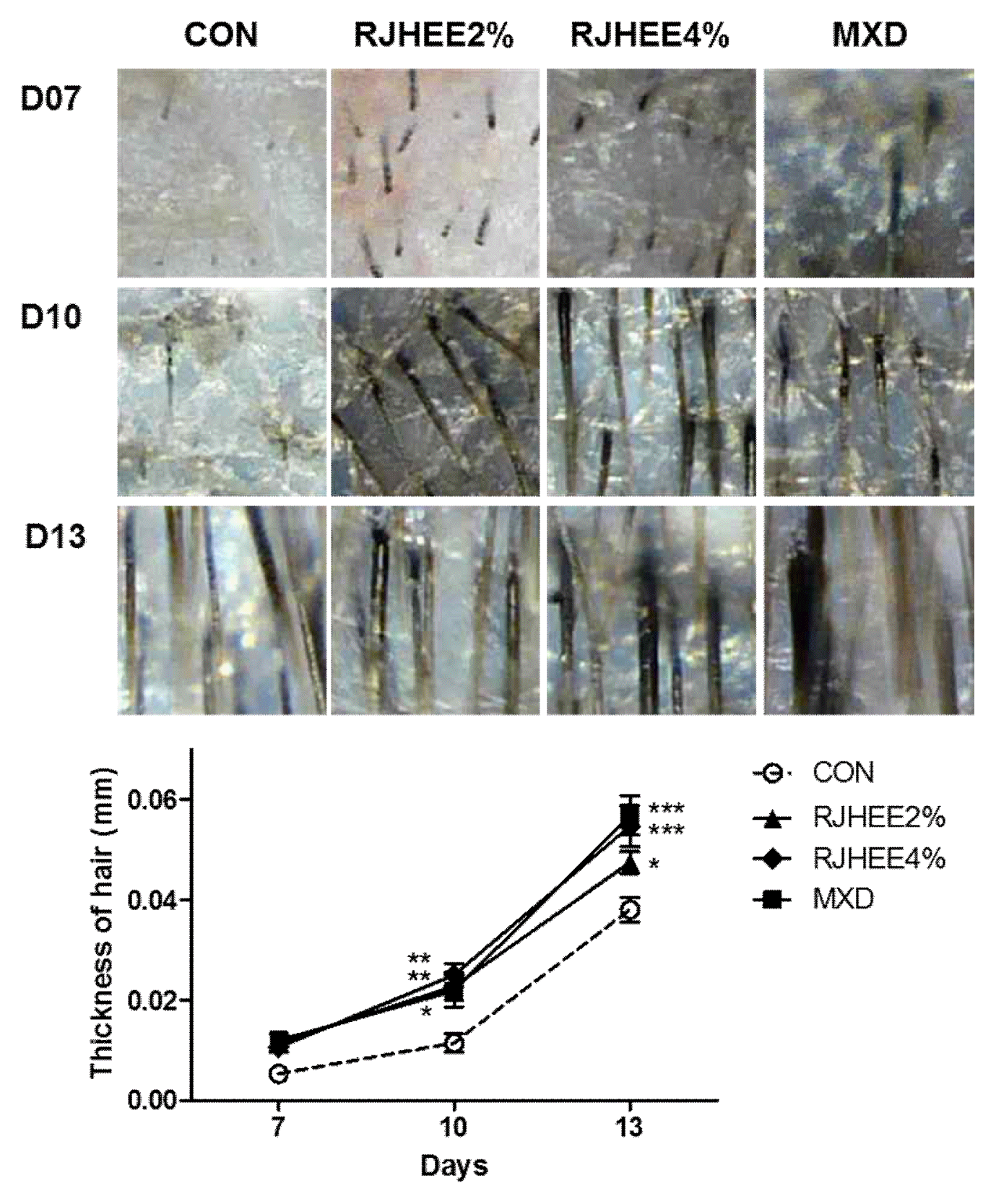
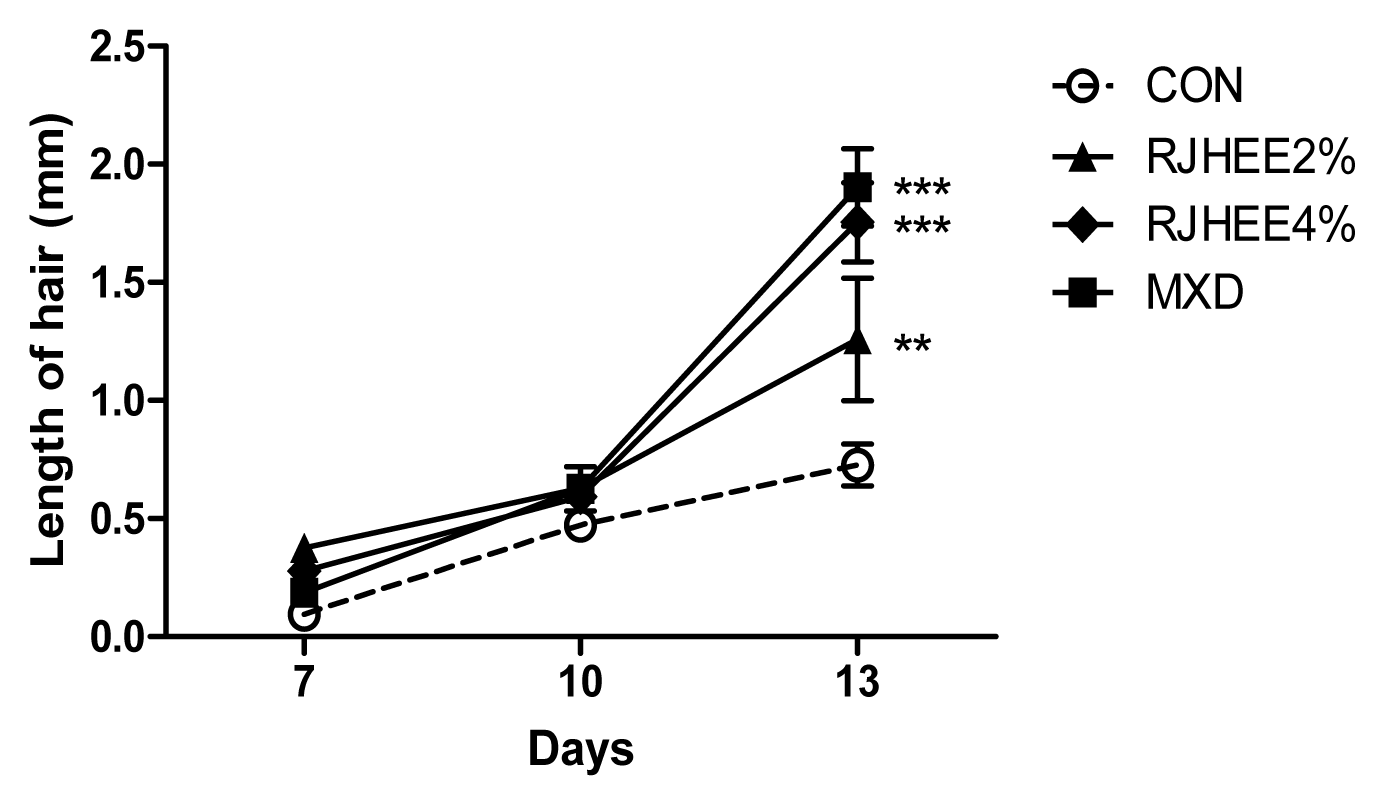
On days 1, 7, 10, 13 and 17 after shaving, the mice were lightly anesthetized with diethyl ether, and changes in hair morphology were photographed by a digital camera (DSLT A57, SONY, Japan) on their backsides. Digital pictures were analyzed using Image J software (National Institutes of Health, Bethesda, MD, U.S.A.) to estimate the ratio of hair regrowth (regrown area/total shaved area) in each mouse. Body weight was measured on days 1, 7, 10, 13 and 17 and hair thickness, density, and length were evaluated in each mouse using the Folliscope (ver. 2.8, Lead M, Korea) on days 7, 10, and 13.
5. Histopathology
After 17 days following anagen induction, mice were anesthetized with diethyl ether, and the tissues of the dorsal skin were excised. Part of the excised tissue was fixed in 4% paraformaldehyde (Sigma, St. Louis, MO, USA) for 24 h and, embedded in paraffin, and 5-μm sections were prepared. The sections were deparaffinized in xylene, rehydrated, and stained with Hematoxylin and Eosin (H&E). The sections were examined under a light microscope (Olympus).
6. Western blotting
The skin tissue of mouse was homogenized with ice cold lysis buffer, which consisted of 20 mmol/l Tris HCl (pH 8.0), 150 mmol/l NaCl, 2 mmol/l ethylenediami-netetraacetic acid, 1 mmol/l NaF, 1% Igepal CA 630, 1 mmol/l phenylmethylsulfonyl fluoride, 1 mmol/l Na3VO4, and protease inhibitor cocktail. Following a 10 min incubation on ice, the homogenized suspension was centrifuged at 2,000 x g for 15 min at 4°C, and the supernatant was used to determine protein concentrations. The protein concentration in the tissue lysates was determined using a protein assay kit (Bio Rad Laboratories, Inc., Hercules, CA, USA). Total proteins (20 μg from each sample) were then separated by l0% sodium dodecyl sulfate polyacrylamide gel electrophoresis and were transferred to nitrocellulose transfer membranes (Whatman; GE Healthcare Europe GmbH, Freiburg, Germany). The membranes were blocked with 5% skim milk in TBS Tween (TBST) buffer [10 mmol/l Tris HCl (pH 7.5), 150 mmol/l NaCl and 0.05% Tween 20) for 1 h, and were then incubated with total antibodies to Wnt3α, β-catenin, ERK, Bcl-xL, Bcl-2, Bax, and β-actin and phospho specific antibodies to ERK and GSK3β. The membranes were incubated with the primary antibodies (diluted in 5% skim milk in TBST) overnight at 4°C and were then washed. The membranes were subsequently incubated for 1 h at room temperature with horseradish peroxidase conjugated anti mouse IgG or anti rabbit IgG anti-bodies (diluted in 5% skim milk in TBST). Immunoreactive bands were developed using enhanced chemiluminescence (ECL) regents (Pierce ECL Western Blotting Substrate; Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol.
7. Cell culture
Human keratinocytes (HaCaT) were cultured in RPMI-1640 supplemented with 10% FBS and penicillin/streptomycin (100 units/ml and 100 μg/ml, respectively) at 37oC in a 5% humidified CO2 incubator. The culture medium was passaged every 3 days.
8. MTT assay
Cell proliferation and H2O2-induced oxidative toxicity were determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For cell proliferation assay, HaCaT cells (1.5×104cells/ml) were seeded into 96 well plates, and then treated with RJHEE (0.1, 0.2, and 0.4 mg/ml), 0.01 μ Mminoxidil (MXD) or RJHEE plus MXD for 5 days. MTT solution was then added to each well and the plates were incubated for 4 h.
For H2O2-induced oxidative toxicity, HaCaT cells (5×104 cells/ml) were seeded in 96 well plates. RJHEE (0.1, 0.2, and 0.4 mg/ml) was pretreated in the wells of plates for 24 h and followed by 1 mM H2O2 for 30 min treatment. MTT solution was then added to each well and the plates were incubated for 4 h. The crystallized formazan was dissolved in DMSO and the absorbance was measured at 540 nm by a microplate reader, Spectramax M2 (Molecular Devices, LLC, Sunnyvale, CA, USA).
9. Reactive oxygen species (ROS) assay
HaCaT cells were seeded on black 96-well plate at density of 1.5×105 cells/ml. Cells were stained with 100 μM DCF-DA in Hank’s balanced salt solution (HBSS) for 30 min. The cells were then washed once with HBSS and treated RJHEE (0.1, 0.2 and 0.4 mg/ml for 30 min. Then, the cells were washed once and treated 1 mM H2O2 for 30 min. The fluorescence intensity was determined at 485 nm excitation and 530 nm emission using a fluorescence microplate reader (SpectraMax M2, Molecular Device, LLC, Sunnyvale, CA, USA).
10. Statistical analyses
Statistical analysis was performed using GraphPad Prism ver. 5.01(GraphPad Software, Inc., CA, USA). All data are presented as mean ± standard error (SE). Body weight and hair density, thickness, and length levels were compared using two-way ANOVA at each time point with post-hoc analysis by Bonferroni corrections. Cell viability, proliferation, and ROS levels were compared using one-way ANOVA with post-hoc analysis by followed by Dunett’s post hoc test. p<0.05 were considered to be statistically significant.
Results
1. RJHEE enhances hair growth in C57BL/ 6Nmice
To evaluate the hair growth-promoting activity of RJHEE, we topically applied RJHEE onto the depilated back of C57BL/6 mice daily for 17 days. MXD was separately applied as a positive control. We evaluated the extent of hair growth by observing the skin color at intervals of three or four days. On 7 days, RJHEE group showed partly pale grey color and minute hair in the depilated skin, while less visible grey color was observed in CON group. On 10 days, RJHEE group showed partly dark gray/black color and short hair in the depilated skin, while visible pale gray color was observed in CON group. On 13 days, RJHEE2% and RJHEE4% group showed markedly hair growth to a similar extent as MXD, whereas CON group showed relatively less hair growth. At 17 days, in RJHEE2% and RJHEE4% group, the mice back were covered with overall hair and Con group also showed progressive hair growth. In MXD group, the change in the color of back skin was earlier than in the other groups and covered completely after 17 days(Fig. 1a). The effect on hair growth of RJHEE treatment was assessed by measuring regrown area and shaved area. MXD group showed 92.83% of hair growth rate at 17 days compared with CON group which kept showing 76.49% of hair growth rate. Although less effective than MXD group, RJHEE group exerted 84.36% and 90.02% of hair growth rate at 2% and 4% at 17 days(Fig. 1b). However, there was no difference among groups during experimental periods(Fig. 1c).
2. RJHEE increase hair density, thickness, and lengthin C57BL/6N mice
Hair density, thickness, and length were examined by folliscope. Fig. 2 shows dermoscopic images of hair density at the 7, 10, and 13 days after depilation. Hair density was higher in the 2% and 4% RJHEE group and MXD group than in CON group. A significant difference in hair density was observed at 10 and 13 days(Fig. 2). Hair thickness was also larger in the 2% and 4% RJHEE group or MXD group than Con group at 10 and 13 days(Fig. 3). Hair length was shown no difference among groups until 10 days; however, hair length was longer in the 2% and 4% RJHEE group or MXD group than Con group at 13 days(Fig. 2).
3. RJHEE enhances the formation of hair follicles
Histological analysis of dorsal skin tissues was conducted to define the effect of RJHEE on hair growth. RJHEE group increases the number, size, depth, and length of hair in dorsal skin tissues. The promoting effect of RJHEE on the hair development was confirmed visually by the histological analysis. The numbers of hair follicles were much larger in RJHEE group than that of Con group. The observation indicated that hair cycle was accelerated in RJHEE group compared to Con group, representing normal murine hair cycle pattern(Fig. 5). These findings suggested that RJHEE could contribute to accelerating the development of hair follicles.
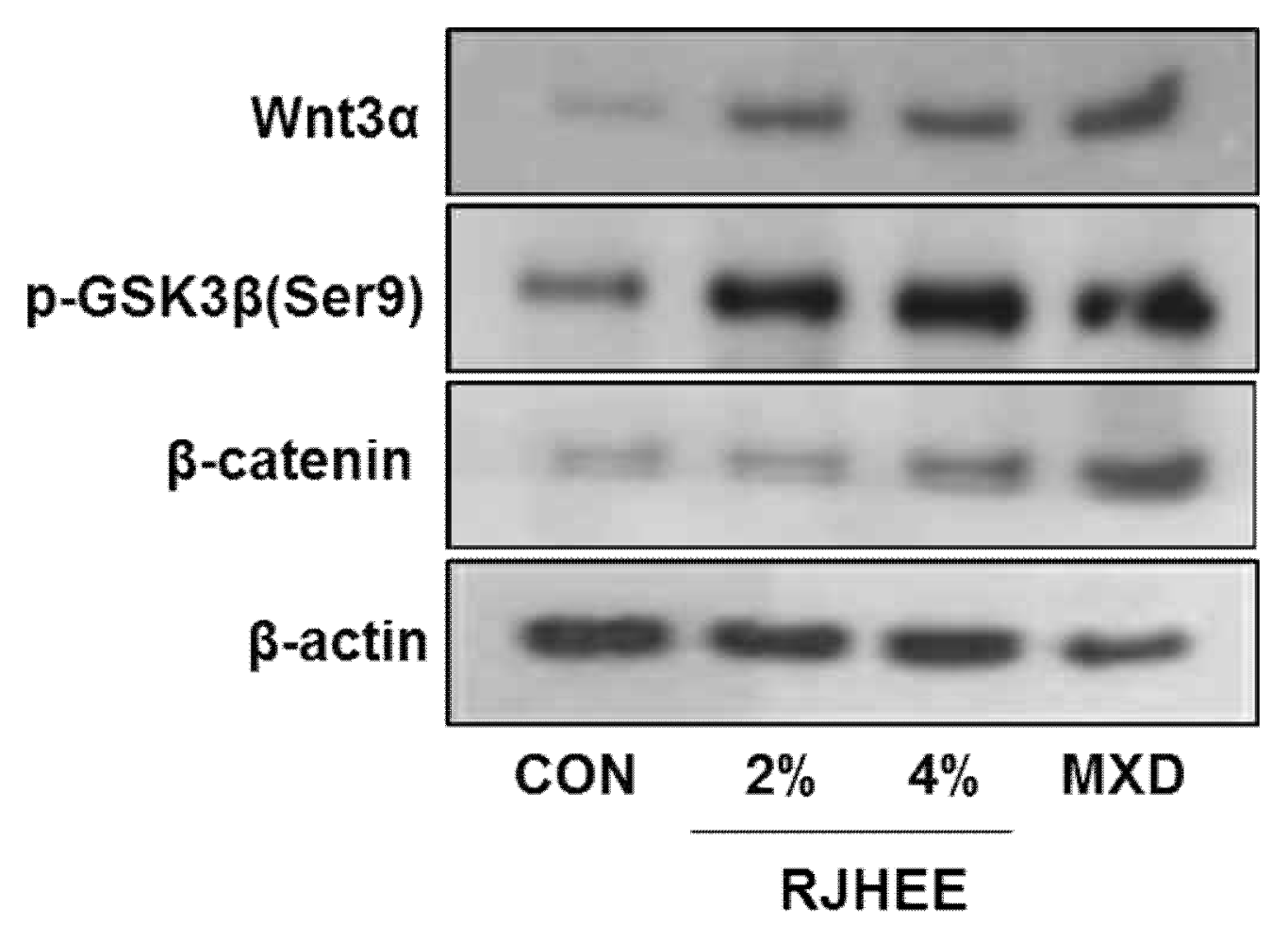
4. RJHEE upregulates activation of β-catenin and Wnt3α expression in the skin of C57BL/6N mice
To elucidate the mechanism underlying the induction of anagen stage in the RJHEE group, we performed western blot analysis using antibodies specific to Wnt3α, p-GSK3β and β-catenin, key molecule in tissue generation and anagen induction in the dorsal skin of C57BL/6 mice.
RJHEE group increased the expressions of Wnt3α, p-GSK3β and β-catenin compared to CON group. In CON group, Wnt3α, p-GSK3β and β-catenin was very weakly detected in skin tissues. However, its expression was increased in the skin of RJHEE group in dose-dependent manner(Fig. 6).
These results suggest that RJHEE can activate the development of hair follicles and the ingression into the anagen stage via Wnt3α, p-GSK3β and β-catenin signaling pathways.
Taken together, these findings suggest a potent role for RJHEE group in the hair-promoting activity related to hair morphogenesis.
5. RJHEE induces the expression of proteins related to cell survival in the skin of C57BL/6N mice
We investigated whether the expression of genes related to cell survival and apoptosis, ERK, Bax, Bcl-xL, and Bcl-2, was involved in promoting effect of RJHEE groups on hair growth.
RJHEE2% group increased the phosphorylation of ERK and the expressions of Bcl-xL and Bcl-2 compared to CON group. RJHEE4% group increased the phosphorylation of ERK and the expressions of Bcl-2 compared to CON group, and reduced the expression of Bax. MXD group increased the phosphorylation of ERK and the expressions of Bcl-xL and Bcl-2 compared to CON group, and reduced the expression of Bax. These findings suggest RJHEE might prevent apoptosis of cells involved in hair grow in skin.
Taken together, these findings suggest a potent role for RJHEE group in the hair-promoting activity related to hair morphogenesis.
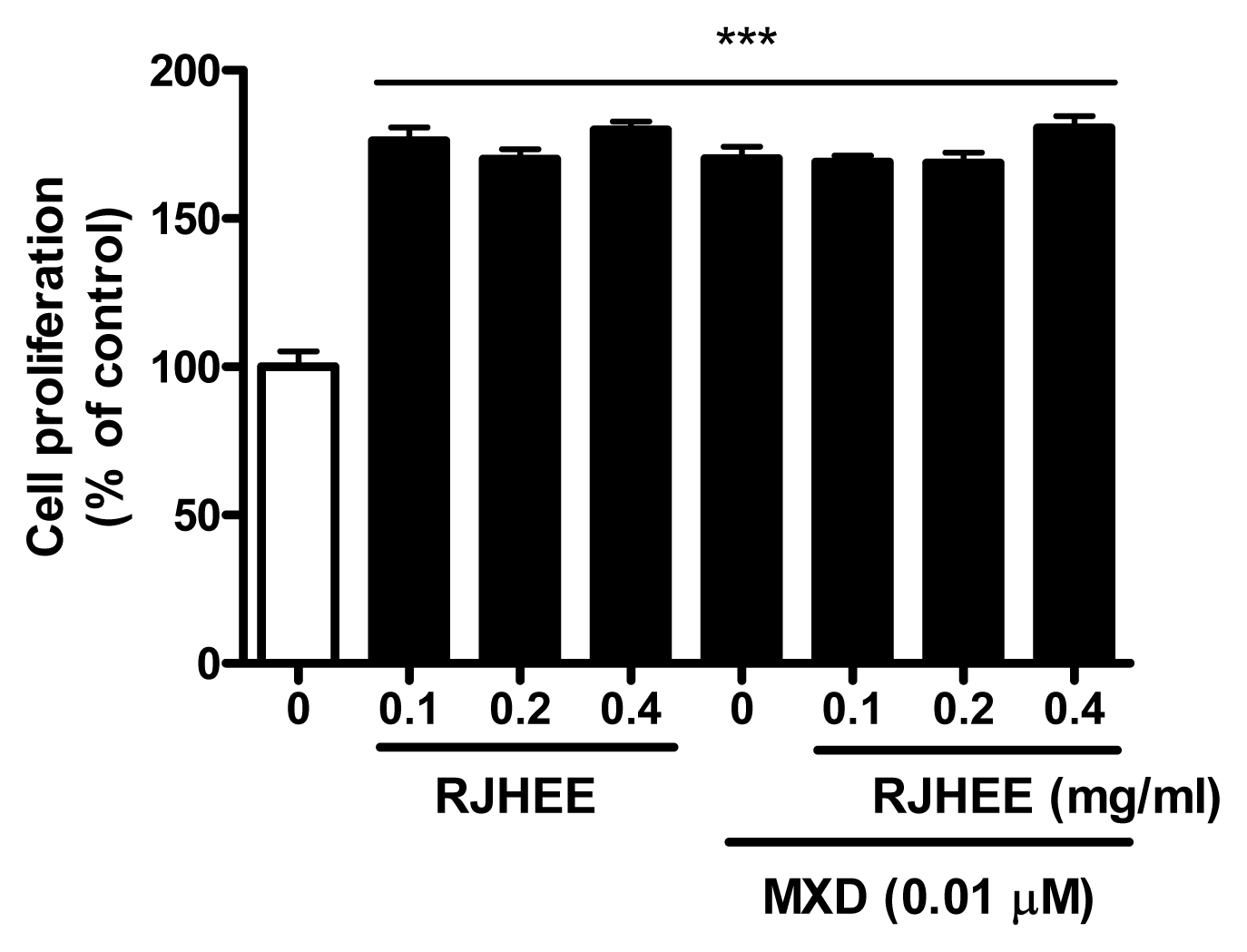
6. RJHEE promotes proliferation of HaCaT cells
RJHEE (0.1, 0.2, and 0.4 mg/ml) significantly increased the proliferation of HaCaT compared with the vehicle-treated control. RJHEE (0.1, 0.2, and 0.4 mg/ml) plus MXD (0.01 μM) also increased the proliferation of HaCaT compared with the vehicle-treated control. However, The effect of the RJHEE (0.1, 0.2, and 0.4 mg/ml) in combination with MXD (0.01 μM) on the proliferation of HaCaT was not significantly greater than that of MXD alone or RJHEE alone(Fig. 8).
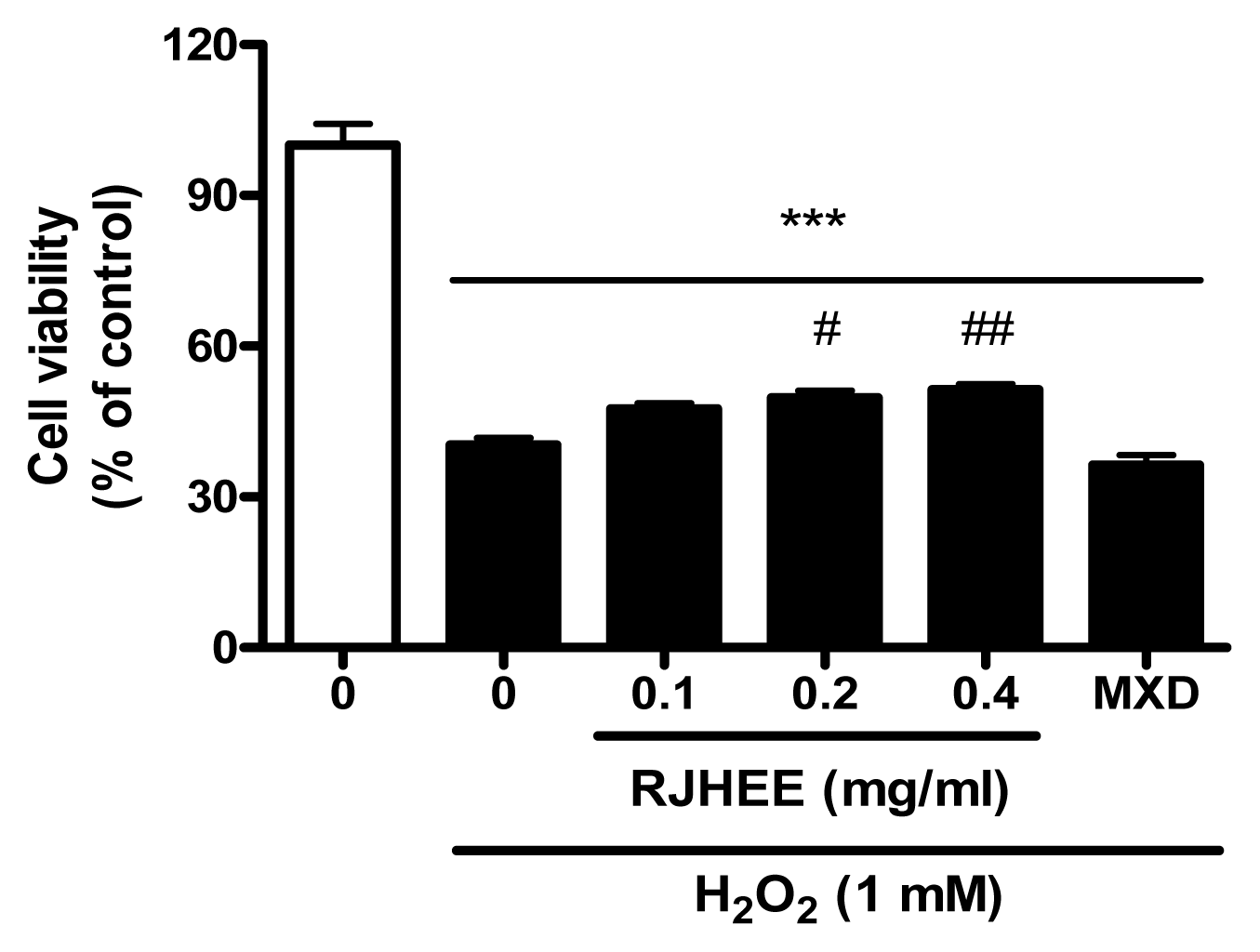
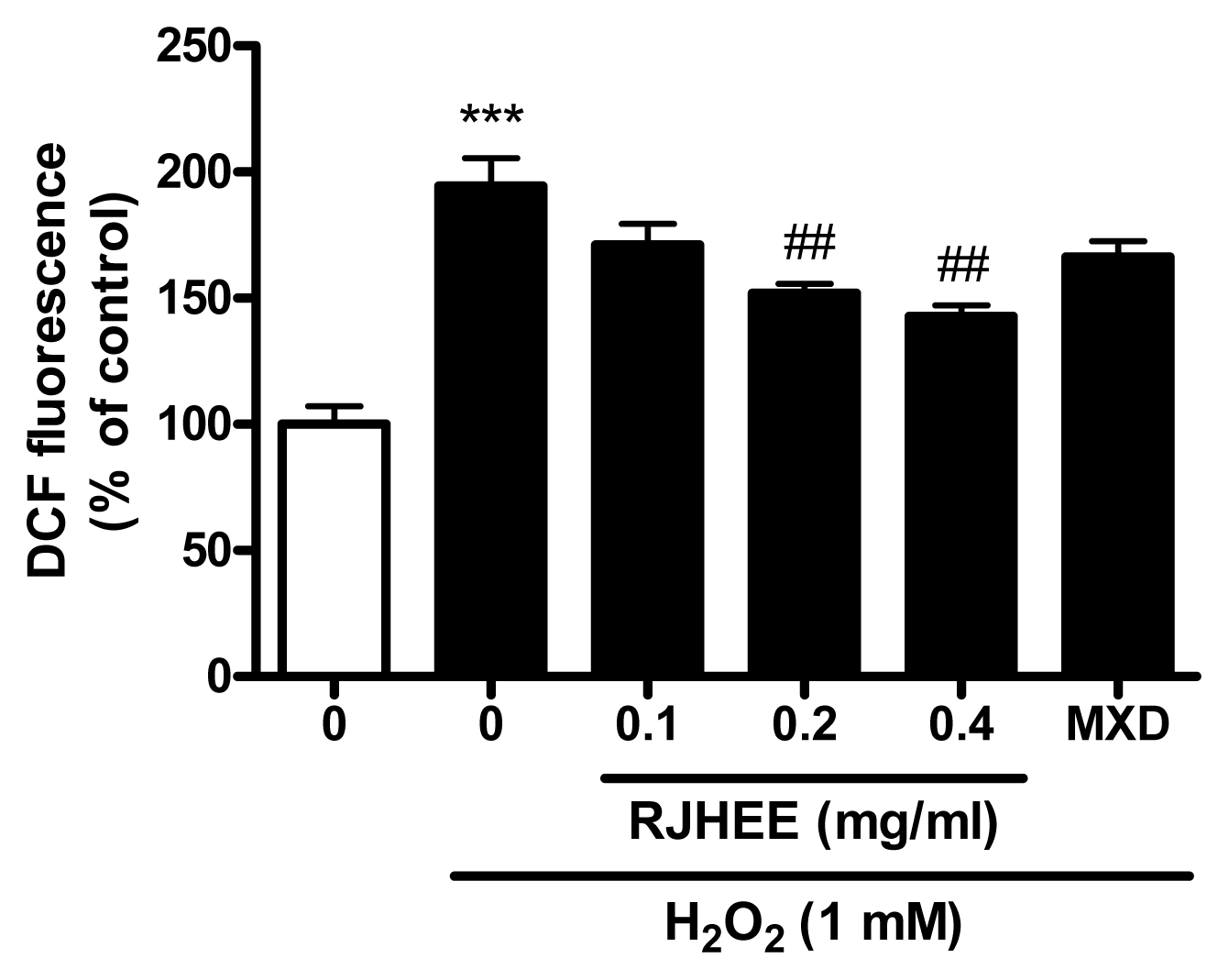
7. RJHEE suppresses H2O2-induced oxidative toxicity and reactive oxygen species (ROS) generation in HaCaT cells
To evaluate oxidative toxicity induced by H2O2, we performed cell viability assay and ROS assay in HaCaT cells. As shown in Fig. 8, RJHEE (0.1, 0.2, and 0.4 mg/ml) did not show cytotoxicity in HaCaT cells. For cell viability assay, HaCaT cells with or without pretreatment with RJHEE (0.1, 0.2, and 0.4 mg/ml) or MXD (0.01 μM) for 24 h were exposed to 1 mM H2O2 for 30 min. Exposure to H2O2 (1 mM) induced cell death of HaCaT cells and ~60% of the cells were dead after 30 min exposure. In contrast, cells treated with RJHEE (0.1, 0.2, and 0.4 mg/ml) showed cell viability of 47.53%, 49.72%, and 51.33% respectively. However, MXD (0.01 μM) showed no difference compared to H2O2 alone on cell viability(Fig. 9). For ROS assay, HaCaT cells with or without pretreatment with RJHEE (0.1, 0.2, and 0.4 mg/ml) or MXD (0.01 μM) for 30 min were exposed to 1 mM H2O2 for 30 min. Exposure to H2O2 (1 mM) induced ROS generation of 194.5% after 30 min exposure. In contrast, cells treated with RJHEE (0.1, 0.2, and 0.4 mg/ml) showed ROS generation of 171.1%, 152.0%, and 142.8% respectively. However, MXD (0.01 μM) showed no difference compared to H2O2 alone on cell viability(Fig. 10).
Discussion
RJH is a multiyearold plant belonging to Polygonaceae. It grows naturally all over our country and grows well in humid places such as forests and streams15). RJH traditionally has been used in diverse skin diseases as an herbal medicine for external application in East-Asia region6). It has antioxidant effect on human skin diseases. It is a native plant in Okinawa, Japan and has traditionally been used by local people for the treatment of acute and chronic skin diseases16). Despite such many uses, in the past they did not have enough understanding of the pharmacological effects of RJH. In recent years, research on the pharmacology and components of RJH has been actively conducted. About the content of RJH, Jang’s17) research has reported that 24-nor-Ursane type triterpenoids is contained in the stem, and Jiang18) and Chen19) have been studied the components which are separated from the roots. The antimicrobial activity of RJH also has been studied20), and antioxidant effects have been studied21). It has been reported that RJH has treats symptoms of dermatosis in oriental medicine literature. Experiments have been conducted to demonstrate the therapeutic effect of atopic dermatitis. Ahn’s reported on the treatment effect of RJH has hydrothermal extract on atopic dermatitis22) and Lee studied on the inhibition effect of atopic dermatitis23).Not only in the recent studies of RJH has, but also widely used remedy for a long time ago. According to the records of Compendium of Materia Medica (本草綱目), it is effective for skin diseases such as swollen area and swelling, and it is reported that there is no toxicity and no taste, and it is reported that it is effective for constipation, indigestion and intestinal bleeding in the private sector. According to the records of Donguibogam: Principles and Practice of Eastern Medicine(東醫寶鑑), it is reported that it is used as an external medicine because it treats scabies, ringworm, furuncle, and hemorrhoids and has an insecticidal effect. Based on the above results, it was suggested that of RJH could be used as an effective herbal medicine for hair loss caused by scalp dermatitis.
This study was performed to evaluate hair promoting effect using C57BL/6 mice model and keratinocyte protecting effect of RJH using HaCaT cells. Depilated C57BL/6 mice are a useful model for hair research and HaCaT cells, human keratinocyte as a main component of skin plays integrated activities in hair morphogenesis.
In this study, RJHEE significantly induced hair growth involving hair density, thickness, and length. Histologic observation showed RJHEE application induced differentiation and proliferation of hair follicle. We identified that these effects of RJHEE are induced via the regulation of Wnt/GSK-3β/β-catenin, ERK, and Bcl-2 anti-apotosis signaling pathway. The regulation of Wnt/GSK-3β/β-catenin pathway is important for entry and maintenance to the anagen stage of hair follicles24–26). Wnts binding to Frizzled receptors prevents phosphorylation of β-Catenin by inhibiting a complex including GSK3. Accumulating β-catenin translocated to the nucleus, where it can bind TCF and stimulate genes associated to hair growth27,28). It has been reported that minoxidil promotes the proliferation of hair follicles through MAPK kinase (ERK, JNK, and p38) signaling pathways29,30). It has been reported that increased cell apoptosis in the hair cells precedes the transition into catagen stage from anagen stage in hair cycle and the Bax/Bcl-xL/Bcl-2 are expressed in the hair cells of which are involved in hair growth31,32).
Hair follicles are miniorgans consisted by keratinocytes and its mesenchyme of dermal papilla. Its cycle changes by also oxidative stress, not only aging and hormonal stimuli33).
Oxidative stressinduces an imbalance between the production of ROS and ROS-induced cellular damage34,35). Although hair follicles are considered relatively resistant to ROS, lasting exposure to excessive intrafollicular ROS could provide one explanation for hair loss.
Excessive intrafollicular ROS accumulation has been expected to cause senescence of follicular cells and androgenetic alopecia35,36), and inflammation associated with these conditions is further increased by the oxidative activities of immune cells and secrete H2O2 37).
Keratinocytes (HaCaT) models are important in the growth of hair because keratinocytes are part of the hair follicle and hair shaft, and produce keratin, the major protein of hair38–41).
In this study, RJHEE promoted the proliferation of keratinocytes and suppressed H2O2-induced oxidative toxicity and ROS generation of keratinocytes. These results suggest that RJHEE has an efficacy on hair growth and proliferation of hair follicle.
Conclusion
In this study, we obtained the following results for RJHEE: (1) RJHEE showed acceleration of hair growth and the proliferation of hair follicles in mouse model; (2) RJHEE activated the expression of Wnt/β-catenin and the phosphorylation of GSK-3β in mouse skin tissue; (3) RJHEE increased the phosphorylation of ERK and the expression of Bcl-2 in mouse skin tissue; (4) RJHEE promotes the proliferation of HaCaT cells and inhibits H2O2-induced toxicity and ROS generation in HaCaT cells. From these results, it was considered that RJHEE have the potential effect to promote the hair growth and delay the start of stage of catagen. Therefore, it is suggested that RJHEE might be a good candidate for helping hair growth promotion.