Introduction
Cholangiocarcinoma (CCA) defines as bile duct cancer occurring in the intrahepatic, perihilar, or distal biliary tree, excluding gallbladder or ampulla of Vater. Hepatobiliary malignancies account for 13% of cancer-related deaths globally, and 10%–20% of these are attributable to CCA1,2. Although CCA is the most common biliary malignancy and the second most common hepatic malignancy, it shows poor prognosis due to the lack of specific symptoms and high invasiveness which often cannot be curable by surgical treatments3,4. Moreover, CCA cells do not respond or weakly respond to chemotherapy or radiotherapy5, thus researches for therapeutic herbal medicines are required. Particularly, investigation of therapeutic herbs for intrahepatic cholangiocarcinoma (iCCA) is earnestly needed since epidemiologic studies have indicated that the age-adjusted mortality rate of iCCA is increasing, whereas the mortality rate of perihilar CCA and distal CCA could be decreasing2,6–10.
CCA develops from the accumulation of genetic and epigenetic alterations in regulatory genes in cholangiocytes11–13. To prove the anti-tumor effect of a herbal medicine, investigation of apoptosis mechanism has been considered as valid method, since defects in apoptotic pathways are believed to contribute to malignancy formation14,15. Apoptosis is essential mechanism for normal development, host defense, and suppression of oncogenesis, and it can be explained as a programmed cell death involving the degradation of cellular components by cysteine proteases called caspases16,17. These caspases can be activated through either the mitochondria-mediated intrinsic pathway or death receptor-mediated extrinsic pathway. Investigation of molecular mechanism of apoptosis seems necessary, as activation of these pathways is a key mechanism of anti-tumor agents to kill tumor cells18,19.
Orostachys japonicus (O. japonicus) extracts have shown various biological activities including anti-inflammatory20, neuroprotective21, anti-ulcerative22, anti-oxidant23, and anti-tumor effects24–26. Particularly, it has been recognized as a potential anti-tumor treatment for a variety of tumor cells in Korea. In the previous studies, the extract of O. japonicus showed anti-tumor effect via inhibition of cell proliferation and induction of apoptosis in human hepatic stellate cells27, leukemia cells28,29, human colon cancer cells30, and human prostate cancer cells31.
In spite of the therapeutic suggestion of O. japonicus, the anti-tumor effect against CCA has not been studied yet. Considering the increasing mortality rate, iCCA cell line was utilized as a subject of our investigation. SNU-1079 is human biliary tract cancer cell line from intrahepatic duct32, which has been used to investigate the anti-tumor effect of Korean herbal medicines33,34. Thereby it was regarded as an appropriate model to investigate the anti-tumor effect of O. japonicus against iCCA.
In the present study, O. japonicus DW and EtOH extracts were treated to SNU-1079 cell line to investigate the capability as an anti-tumor agent against CCA. We measured the inhibitory effect on cell proliferation and regulatory effect on apoptosis-related genes and caspase activation to demonstrate by which molecular pathway O. japonicus functions to induce apoptosis.
Materials and Methods
1. Preparation of Orostachys japonicus A. Berger extract (DW & EtOH)
After purchased from Kyung Hee Korean Herbal Medicine Research Center, Seoul, Korea, O. japonicus was cut down in a proper size and used for extraction procedure.
DW extract 100 g of O. japonicus and 1000 ml Distilled Water were boiled for 2 hours using extractor. The resulting water extract was filtered through a Whatmann Paper, and then vacuum evaporation was proceded by rotary evaporator. After the concentrated extract was freeze-dried (EYELA, Tokyo, Japan), the final weight of the extract was 12.8 g, which was 12.8% of natural product.
EtOH extract 100 g of O. japonicus and 1000ml 50% EtOH were boiled for 2 hours using extractor. The resulting ethanol extract was filtered through a Whatmann Paper, and then vacuum evaporation was proceded by rotary evaporator. After the concentrated extract was freeze-dried (EYELA, Tokyo, Japan), the final weight of the extract was 6.5 g, which was 6.5% of natural product.
2. Cell culture
The human cholangiocarcinoma cell line SNU-1079 was purchased from the Korean Cell Line Bank (Seoul, Korea). The cells were cultured in RPMI 1640 (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin at 37 ºC in a humidified atmosphere of 5% CO2 and 95% air.
3. Cell viability assay
Cell proliferation was evaluated using the CellTiter 96 Aqueous One solution (Promega, Madison, WI, USA). SNU-1079 cells were seeded at a density of 1×104 cells/well in 96-well plate and incubated with various concentrations of O. japonicus DW and EtOH extracts (0, 50, 100, 200 and 300/ml) at 37ºC for 24, 48 or 72 h. Cell viability was determined through a colorimetric assay by using PMS/MTS solution. The absorbance was determined at 492 nm with background subtraction at 650 nm.
4. ELISA assay
The number of apoptotic SNU-1079 cells was measured using the Cell Death Detection ELISAplus kit (Roche Molecular Biochemicals, Manndeim, Germany). Cells (1×104) were incubated with various concentrations of O. japonicus DW and EtOH extracts (0, 100, 200 and 300) for 24, 48 or 72h. They were then lysed with the cell lysis buffer (200 μl). The cell lysates were assayed for DNA fragments by using the cell death ELISAplus according to the manufacturer’s protocol. DNA fragmentation was evaluated at 405 nm against an untreated control.
5. FACS analysis
Cells (5×105) were treated with various concentrations of O. japonicus DW and EtOH extracts (0, 150 and 300) for 24, 48 or 72 h. At the end of the treatment period, the cells were harvested and washed with PBS. They were then fixed with 70% ethanol for 1 h, treated with RNase A (20 μg/ml) at 37 ºC for 1 h and stained with Annexin-V/PI assay kit (BD Biosciences, USA). The apoptosis rate was analyzed using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA).
6. RNA extraction and real-time PCR
Total RNA was purified from cultured cells by using an RNA-Bee solution kit following the manufacturer’s protocol (Tel-Test, Friendswood, TX, USA). First-strand cDNA synthesis was performed with 1 μg of total RNA and transcribed into cDNA using a reverse transcription system with random hexamers according to the manufacturer’s protocol. The primer sequences used were as follows (Table 1):
Real-time PCR was performed using a StepOneplus real-time PCR system with the Power SYBR Green PCR Master Mix (Applied Biosystems, Foster, CA, USA). The PCRs were performed with 1 ml of cDNA in 20 ml reaction mixtures which is consisted of 10 ml Power SYBR Green PCR Master Mix, 2 ml of primers, and 7 ml of PCR-grade water. The reactions were performed with a denaturation step at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and at 60 °C for 1 min. The crossing point of each target gene with β-actin was calculated by using the formula 2−(target gene – ß-actin) and the relative amounts of the PCR products were quantified.
7. Immunoblot analysis
Cells were collected and washed with cold PBS then lysed using lysis buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin] containing 1 mM PMSF (Cell Signaling Technology, Inc., Boston, MA, USA). The protein concentration was determined by a BCA protein assay according to the manufacturer’s protocol. Thirty micrograms of protein were fractionated by 12% SDS-PAGE and transferred by electrophoresis to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk for 1 h at room temperature and then incubated overnight with antibodies against Caspase-3 and β-actin (Cell Signaling Technology), diluted 1:1,000 with Tris-buffered saline containing 0.05% Tween 20 (TBS-T). After washing with TBS-T for 1 h, the membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies diluted 1:2,500 in TBS-T. The membranes were subsequently washed with TBS-T for 1 h, and proteins were detected using Enhanced Chemiluminescence Kit (Santa Cruz Biotechnology, CA, USA). Protein expression was analyzed using Davinch-Chemi™ Chemiluminescence Imaging System (Davinch-K Co. Ltd., Seoul, Korea). The intensities of bands were measured by densitometry (Imagej program, NIH) and expressed as intensities relative to β-actin.
Results
1. Effects of Orostachys japonicus on cell proliferation
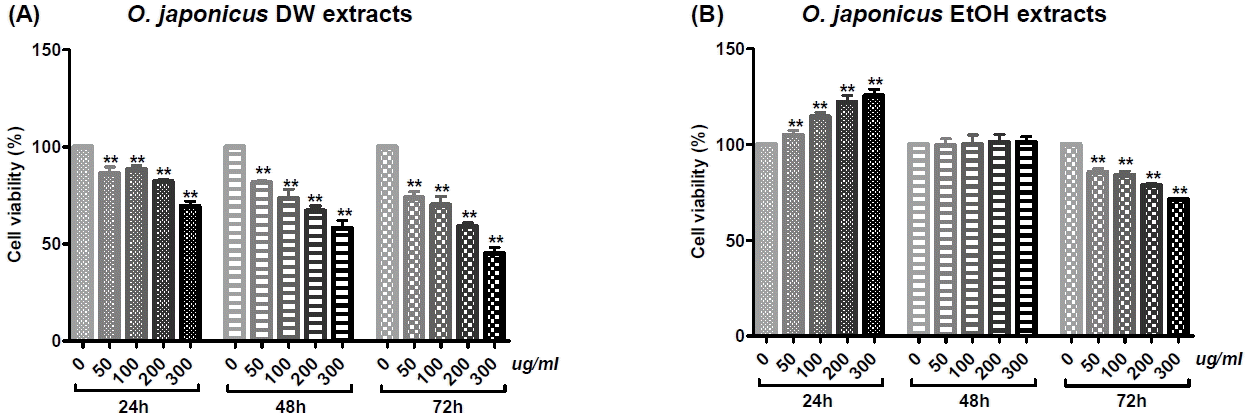
Cholangiocarcinoma SNU-1079 cells were treated with various concentrations of O. japonicus DW and EtOH extracts (0, 50, 100, 200 and 300 μg/ml) for 24, 48 or 72 h. The effect of O. japonicus on cell proliferation was measured by PMS/MTS colorimetric assay. DW extracts of O. japonicus significantly inhibited the proliferation of SNU-1079 cells in a dose- and time-dependent manner (Fig. 1(A)). The 72 h EtOH extracts significantly inhibited cell proliferation in a dose-dependent manner (Fig. 1(B)).
2. Effects of Orostachys japonicus on the apoptosis rate using ELISA assay
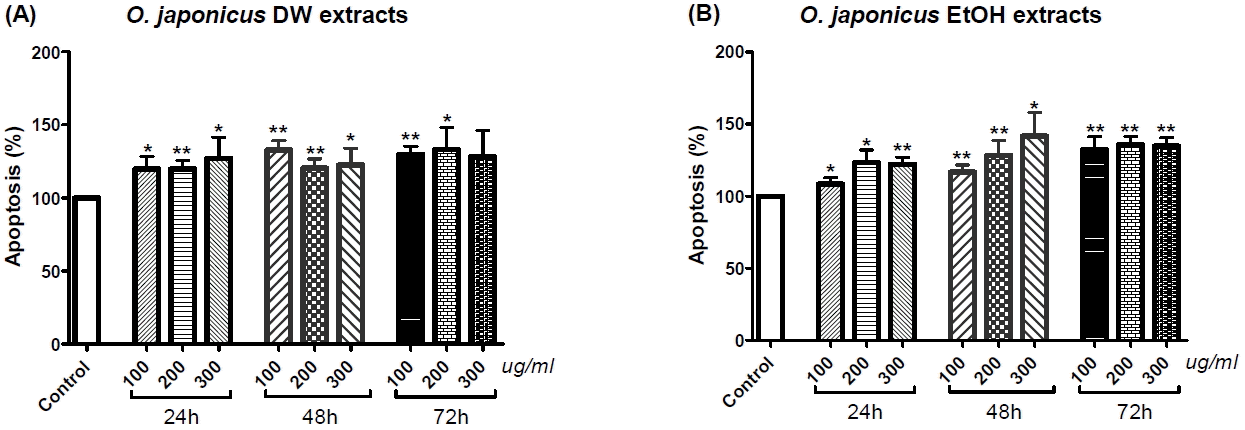
Cholangiocarcinoma SNU-1079 cells were treated with various concentrations of O. japonicus DW and EtOH extracts (0, 100, 200 and 300 μg/ml) for 24, 48 or 72h. Apoptotic cells were detected by cell death detection ELISA. Both DW and EtOH extracts increased the number of apoptotic cells at all concentrations (100, 200 and 300 μg/ml) and times (24, 48 and 72 h), and almost all of the data were statistically significant (Fig. 2(A) and (B)). However, neither dose-dependent nor time-dependent variances were found (Fig. 2(A) and (B)).
3. Effects of Orostachys japonicus on the apoptosis rate using FACS analysis
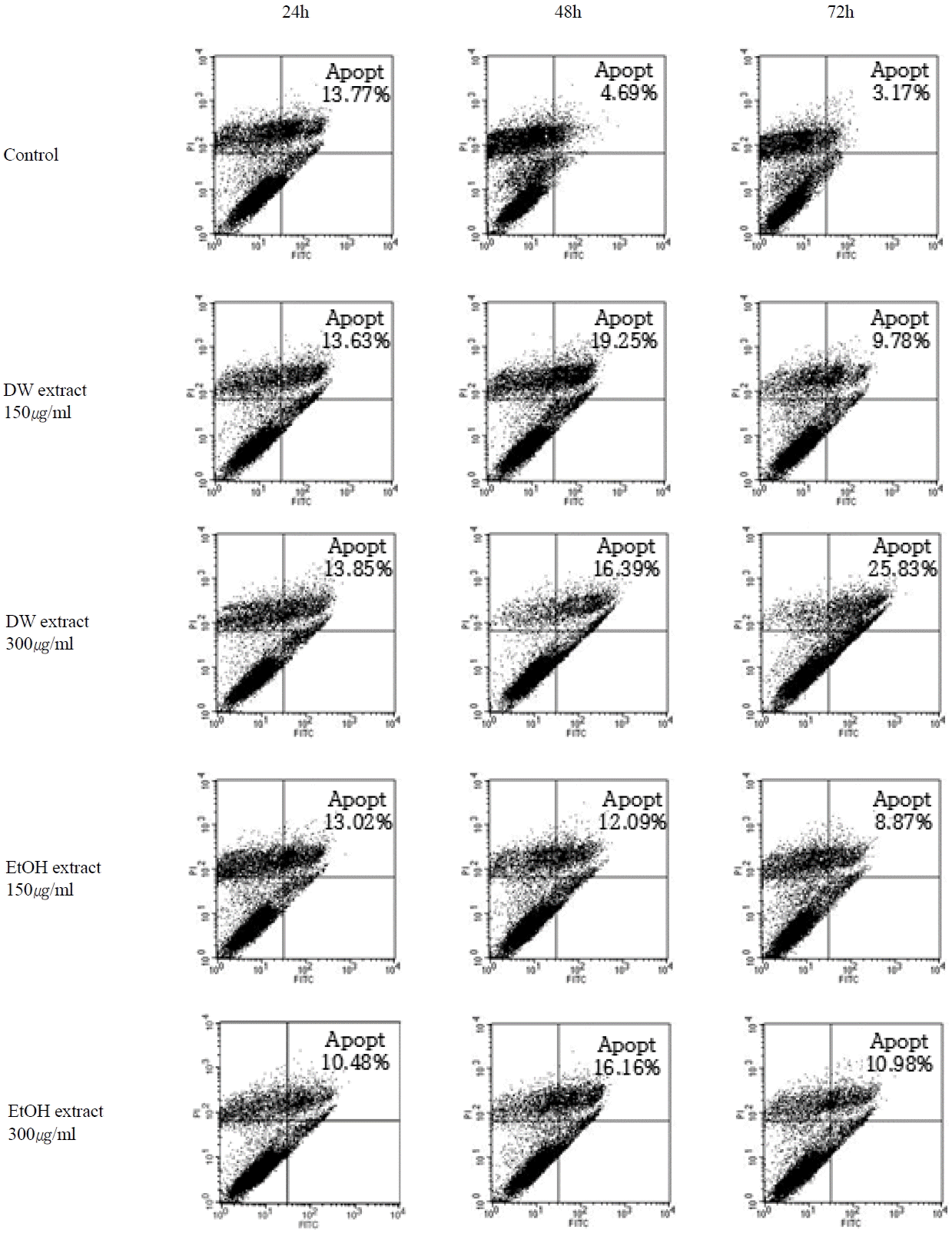
Cholangiocarcinoma SNU-1079 cells were treated with various concentrations (0, 150 and 300 μg/ml) of O. japonicus DW and EtOH extracts for 24, 48 or 72 h, and then analyzed using flow cytometry.
The cells that were undergoing early apoptosis were FITC+PI−, appearing in the lower right quadrant, while cells undergoing necrosis or late apoptosis were FITC+PI+, appearing in the upper right quadrant. The sum of the cells in both areas, was considered as the total number of apoptotic cells.
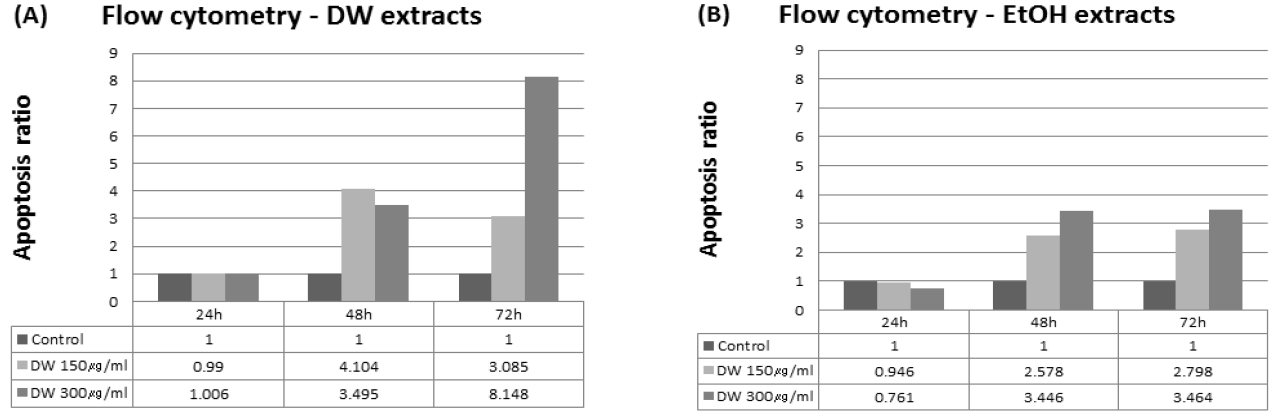
The results indicated an increased apoptosis rate with 48 and 72 h treatment using either DW or EtOH extracts (Fig. 3). There was a dose-dependent increase in apoptotic cells with 72 h DW extract treatment (Fig. 4(A)) and with 48 and 72h EtOH extract treatments (Fig. 4(B)). The highest apoptosis rate occurred with 300 μg/ml DW extract treatment for 72 h (Fig. 4(A)). However, with both DW and EtOH extracts, the effect on apoptosis was not notable at 24 h (Fig. 3 and 4).
4. Effects of Orostachys japonicus on the mRNA expression of apoptosis related genes
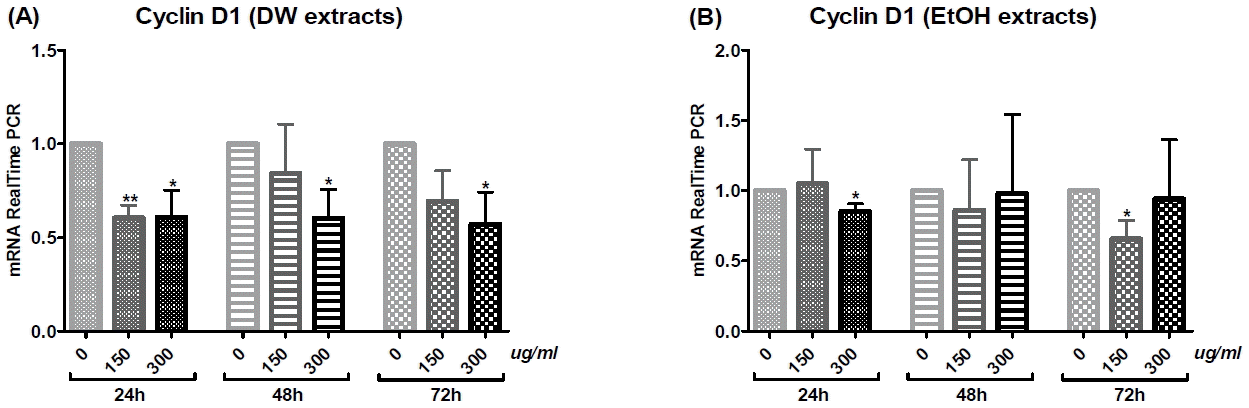
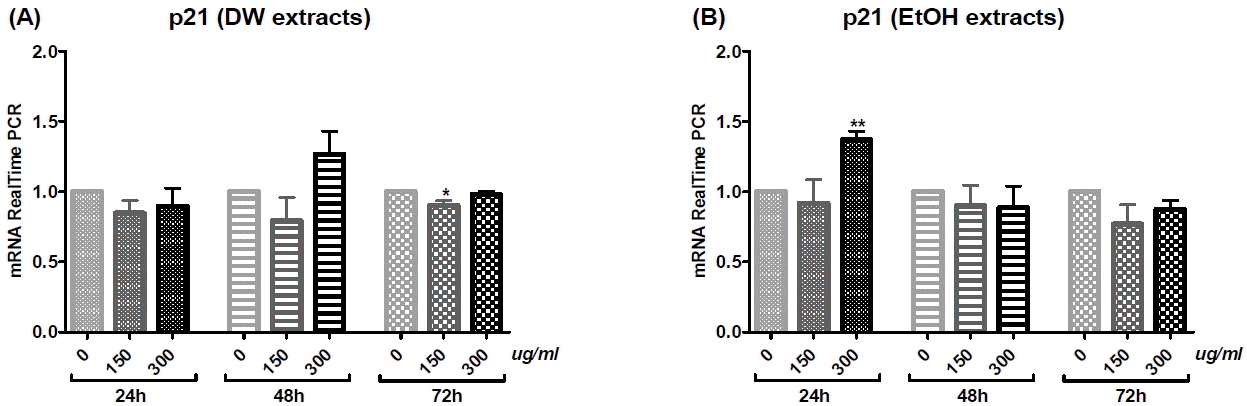
Cholangiocarcinoma SNU-1079 cells were treated with various concentrations of O. japonicus DW and EtOH extracts (0, 150 and 300 μg/ml) for 24, 48 or 72 h. The mRNA levels of apoptosis- and cell cycle progression-related genes (Bcl-2, Mcl-1, Bax, Survivin, Cyclin D1 and p21) were evaluated using real-time PCR to investigate the anti-tumor effect of O. japonicus at the level of gene expression.
O. japonicus DW and EtOH extracts decreased Bcl-2, Mcl-1, Survivin, and Cyclin D1 mRNA levels, with some statistically significant changes observed (Fig. 5, 6, 8, and 9). DW extracts significantly increased Bax mRNA levels at particular treatment concentrations and times (Fig. 7(A)), while EtOH extracts did not produce significant increases in Bax mRNA (Fig. 7(B)). There was no clear correlation between treatment time or concentration with p21 mRNA levels for both DW and EtOH extract treatments (Fig. 10), although treatment with 300 μg/ml EtOH extract for 24 h increased p21 mRNA levels significantly (Fig. 10(B)). Overall, treatment with DW extract was more effective than with EtOH extract.
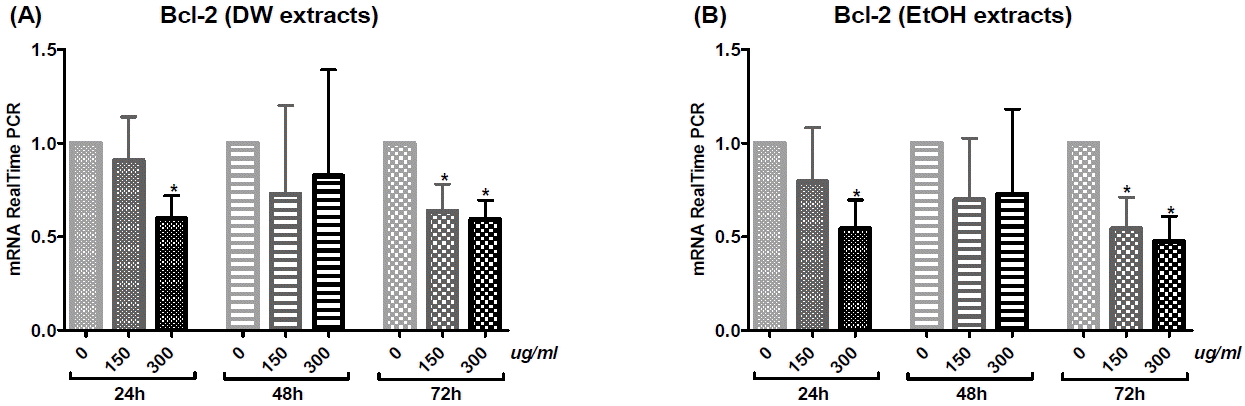
1) Bcl-2
Both DW and EtOH extracts decreased the Bcl-2 mRNA expression in a dose-dependent manner. For both DW and EtOH extracts, treatment at 300 μg/ml for 24 or 72 h, as well as at 150 μg/ml for 72 h, significantly decreased Bcl-2 mRNA levels (Fig. 5).
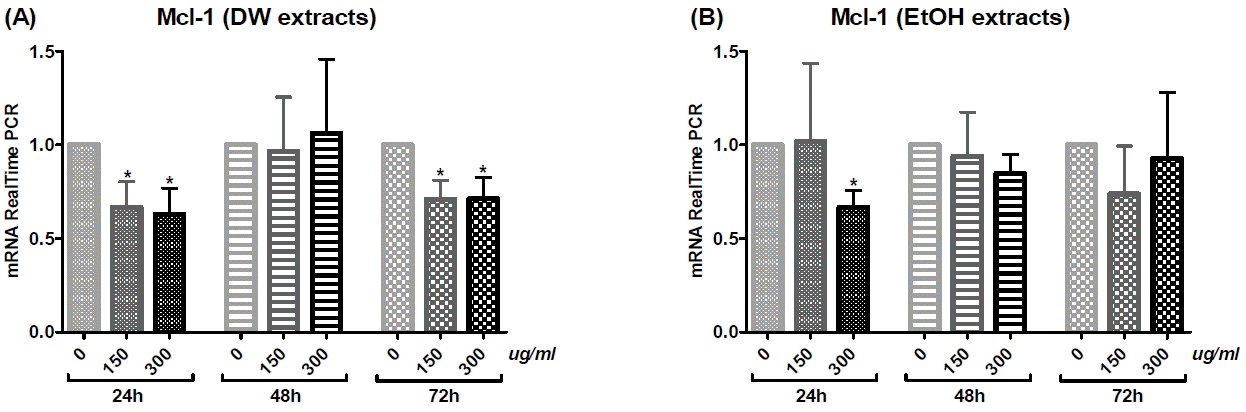
2) Mcl-1
Mcl-1 mRNA levels decreased in a dose-dependent manner with 24 or 72 h DW extract treatment (Fig. 6(A)), as well as with 24 or 48 h EtOH extract treatment (Fig. 6(B)). Treatment with DW extract at doses of 150 μg/ml and 300 μg/ml for 24 and 72 h (Fig. 6(A)), as well as with EtOH extract at a dose of 300 μg/ml for 24 h (Fig. 6(B)) significantly reduced mRNA expression.
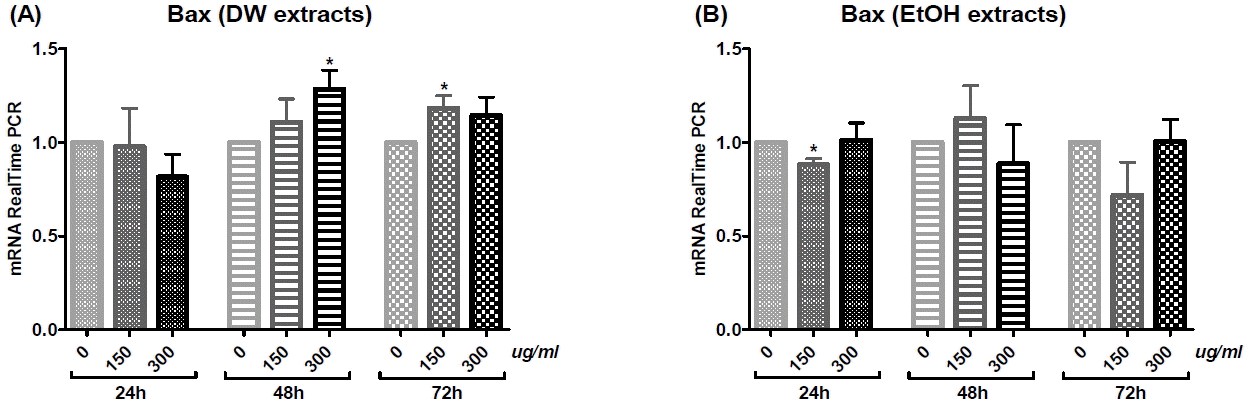
3) Bax
Treatment with DW extract for 48 h increased Bax mRNA expression in a dose-dependent manner (Fig. 7(A)). Treatment with DW extract at a dose of 300 μg/ml for 48 h or 150 μg/ml for 72 h significantly increased Bax mRNA levels (Fig. 7(A)). There was no significant increase in Bax mRNA with EtOH extract treatment (Fig. 7(B)).
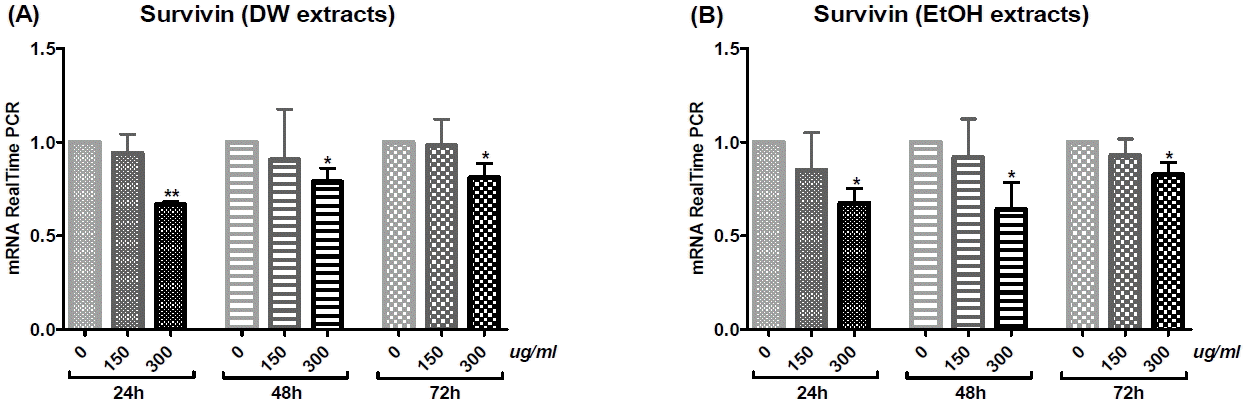
4) Survivin
Both DW and EtOH extracts decreased Survivin mRNA levels in a dose-dependent manner for all treatment times. Particularly, for both DW and EtOH extracts, treatment with a 300 μg/ml dose, for 24, 48 or 72 h significantly reduced Survivin mRNA expression (Fig. 8).
5) Cyclin D1
DW extracts decreased Cyclin D1 mRNA levels in a dose-dependent manner. Treatment with DW extract at a dose of 300 μg/ml for 24, 48 or 72 h, as well as at 150 μg/ml for 24 h, significantly reduced mRNA expression (Fig. 9(A)). Treatment with EtOH extract at a dose of 300 μg/ml for 24 h or at 150 μg/ml for 72 h also significantly decreased Cyclin D1 mRNA levels (Fig. 9(B)). DW extracts were more effective than EtOH extracts.
6) p21
P21 mRNA expression was not notably increased with either DW or EtOH extract treatment (Fig. 10). Treatment with EtOH extract at a dose of 300 μg/ml for 24 h (Fig. 10(B)), as well as with DW extract at a dose of 300 μg/ml for 48 h (Fig. 10(A)) increased p21 mRNA levels, but only the EtOH extract produced a statistically significant induction.
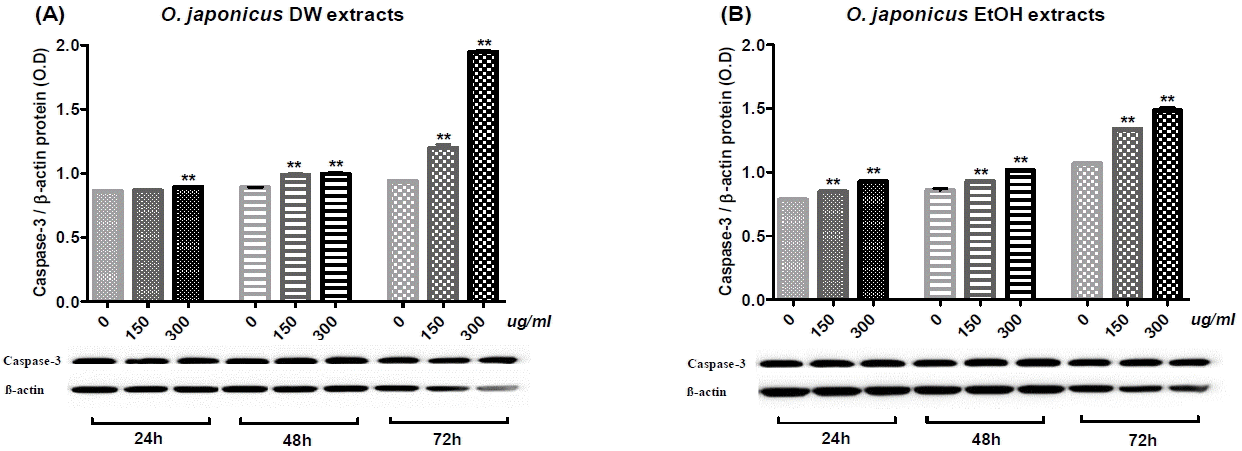
5. Effects of Orostachys japonicus on Caspase-3 activation
Cholangiocarcinoma SNU-1079 cells were treated with various concentrations of O. japonicus DW and EtOH extracts (0, 150 and 300 μg/ml) for 24, 48 or 72 h. To investigate the molecular mechanism of O. japonicus-induced apoptosis, changes in the activation of Caspase-3 protein were examined by immunoblot analysis. Densitometric analyses are presented as the relative ratios of Caspase-3 to β-actin.
Both DW and EtOH extracts increased Caspase-3 activity in SNU-1079 cells in a dose- and time-dependent manner, and almost all of the data were statistically significant (Fig. 11). Particularly, treatment with DW and EtOH extracts for 72 h increased Caspase-3 activation prominently (Fig. 11). DW extract at a dose of 300 μg/ml and the treatment time of 72 h produced the highest induction (Fig. 11(A)).
Discussion and Conclusion
Cholangiocarcinoma (CCA) originates from the malignant transformation of cholangiocytes, the epithelial cells lining the biliary ducts5. CCA is highly fatal as in most patients the cancer is recognized when it is already locally advanced1. Over the past three decades, the overall incidence of CCA appears to have increased, but the percentage of patients who survive more than five years after diagnosis still remains at 10%35,36. An investigation into candidate treatments for intrahepatic CCA (iCCA) seems particularly necessary, as data from the National Cancer Institute Surveillance, Epidemiology and End Results (NCI-SEER) program suggest that approximately 15% of bile duct cancers are iCCAs10,37. Therefore, we utilized an iCCA human biliary tract cell line, SNU-1079, to conduct our study.
Several trials have been made to find potential anti-tumor treatments from a range of Korean medicinal herbs; Orostachys japonicus(O. japonicus) is one of the traditional medicines used as an anti-tumor agent in Korea, which is known to have heat-clearing, dispersing swelling, hemostatic, and dampness-draining effects. In a previous study, O. japonicus inhibited growth in the human hepatic stellate cell line LX2, by inducing apoptosis via MAPK pathway regulated Caspase activation27. Furthermore, other studies have reported that O. japonicus induced apoptosis in human acute promyelocytic leukemia HL60 cells29 and chronic myeloid leukemia K562 cells28, apoptosis and sub-G1 phase cell cycle arrest in human colon cancer SW480 cells30, and cell death in human prostate cancer RC-58T/h/SA#4 cells31. Consequently, we considered O. japonicus as a promising therapeutic against cholangiocarcinoma and further investigated its anti-tumor effects. The process of apoptosis is essential in regulating the homeostasis of the biliary epithelium as it permits the removal of severely damaged cells with non-reversible genomic mutations38. Hence, this study reports the capability of O. japonicus to induce apoptosis.
To evaluate the effect of O. japonicus treatment on cell viability, a PMS/MTS assay was performed. O. japonicus DW extracts inhibited SNU-1079 cell proliferation in a dose-and time-dependent manner (Fig. 1(A)), whereas EtOH extracts inhibited proliferation in a dose-dependent manner but only at 72 h (Fig. 1(B)); this might be related to the doubling time of SNU-1079 cells, which is known to be 72 h. Considering that Korean traditional herbal medicines are usually administered in the form of DW extract, the result suggests that the traditional decoction method is effective for O. japonicus administration. A similar trend also appeared in following experiments.
We investigated the apoptosis rate of SNU-1079 cells with O. japonicus extract treatment, with the reasoning that a defect in the apoptotic process leads to the survival of mutated cholangiocytes, which can undergo a series of other mutations resulting in the malignant transformation of the cells38. ELISA assay and FACS analysis were performed to measure the apoptosis rate. Results from the ELISA assay showed that, O. japonicus significantly increased the apoptosis rate at almost all concentrations and treatment times, but dose- or time-dependent differences were not seen (Fig. 2). This result suggests that both O. japonicus DW and EtOH extracts at doses over 100 μg/ml and treatment times over 24 h long can induce the apoptosis of SNU-1079 cholangiocarcinoma cells. Flow cytometric analysis also showed increased apoptosis rates with O. japonicus extract treatments. The sum of the dead or necrotic cells and cells in apoptosis, was considered as the total number of apoptotic cells. The results suggest that O. japonicus DW and EtOH extracts induce apoptosis in SNU-1079 cells when treated with 150 μg/ml and 300 μg/ml doses for 48 or 72 h (Fig. 3 and 4). Overall, DW extracts were more effective than EtOH extracts, and treatment with DW extract at a dose of 300 μg/ml for 72 h produced the highest apoptosis rate, 25.83% (Fig. 3 and 4(A)). Unlike with the ELISA assay, by FACS analysis O. japonicus DW and EtOH extracts were revealed to be ineffective for 24 h long treatments (Fig. 3 and 4).
To demonstrate the anti-tumor effect of O. japonicus at the gene expression level, we examined the changes in the mRNA expression of apoptosis-related genes in O. japonicus extract treated SNU-1079 cells. Results showed that, O. japonicus DW and EtOH extracts reduced the expression of anti-apoptotic genes Bcl-2 and Mcl-1 (Fig. 5 and 6). The Bcl-2 superfamily of anti-apoptotic proteins is expressed in CCA cells in a high amounts. In CCA cells, Bcl-2 protein exerts its anti-apoptotic activity by preventing cytochrome-c release from the mitochondria thus reducing Caspase-3 activation39. Myeloid cell leukemia-1 (Mcl-1) belongs to the Bcl-2 family, and increases cancer cell resistance to tumor-necrosis-factor (TNF) related apoptosis-inducing ligand (TRAIL)40. The expression of Mcl-1 is also stimulated by bile acids, abundant in the course of cholestasis41. In that, Mcl-1 appears to have an important role in the development of CCA42. Therefore, it is noteworthy that O. japonicus DW extracts significantly decreased mRNA levels of both of these genes in a dose-dependent manner with 24 or 72 h treatment (Fig. 5(A) and 6(A)). Pro-apoptotic protein Bax is believed to induce the opening of the mitochondrial voltage-dependent anion channel (VDAC), thereby releasing cytochrome c and other pro-apoptotic factors from the mitochondria43. Cells treated with O. japonicus DW extracts at a concentration of 150 μg/ml or 300 μg/ml for 48 or 72h exhibited increased Bax mRNA levels (Fig. 7(A)). However, EtOH extracts did not produce notable effects on Bax mRNA expression (Fig. 7(B)). Since Bcl-2 and Bax genes regulate apoptosis through the intrinsic pathway, which is characterized by the permeabilization of the mitochondria and release of cytochrome c into the cytoplasm44, the data suggest that O. japonicus extracts induce SNU-1079 cell apoptosis via the mitochondria-mediated intrinsic pathway by regulating Bcl-2 and Bax expression. Moreover, DW extract seems more effective than EtOH extract.
We also investigated the mRNA expression of cell cycle progression related genes. Survivin is one of the inhibitor of apoptosis (IAP) family proteins, and functions to inhibit caspase activation, thus leading to inhibition of apoptosis or programmed cell death45. Its expression is also highly regulated by the cell cycle as it is only expressed in the G2-M phase. Cyclin D1 is a protein required for cell progression through the G1 phase of the cell cycle46. It is synthesized rapidly during the G1 phase and accumulates in the nucleus. It is degraded as the cell enters the S phase. Overexpression of Cyclin D1 has been shown to correlate with early cancer onset and tumor progression, which can lead to oncogenesis through angiogenesis brought about by increased VEGF production47,48. In this study, O. japonicus DW extracts decreased Survivin and Cyclin D1 mRNA levels in a dose-dependent manner. DW extract at a 300 μg/ml dose significantly inhibited both Survivin and Cyclin D1 mRNA expression for all treatment times, 24, 48 and 72 h (Fig. 8(A) and 9(A)). Similar to DW extracts, EtOH extracts also significantly decreased Survivin mRNA levels in a dose-dependent manner, in cells treated with 300 μg/ml of extract for 24, 48 or 72 h (Fig. 8(B)), whereas the effect on Cyclin D1 expression was not dose-dependent (Fig. 9(B)). P21 (CIP1/WAF1) is a potent cyclin-dependent kinase inhibitor (CKI). This protein inhibits the activity of cyclin-CDK2, -CDK1, and -CDK4/6 complexes, thus it functions as a regulator of cell cycle progression at the G1 and S phase49. We expected O. japonicus to increase p21 mRNA levels, but the results generally did not show this tendency (Fig. 10). The mRNA levels of this gene only increased when SNU-1079 cells were treated with 300 μg/ml of DW extract for 48 h (Fig. 10(A)) or 300 μg/ml of EtOH extract for 24 h (Fig. 10(B)). These results indicate that, O. japonicus regulates cell cycle progression by regulating Survivin and Cyclin D1 mRNA expression levels, but its association with p21 is not understood completely. Regarding the known fact that Survivin is only expressed in the G2-M phase, and Cyclin D1 is required for progression through the G1 phase of the cell cycle, we can assume that the effect of O. japonicus might function by regulating the G1-G2-M phases of cell cycle progression. Further studies are needed to confirm this hypothesis.
To find out molecular pathway of apoptosis, we evaluated Caspase-3 activation in SNU-1079 cells with O. japonicus extract treatments using immunoblot analysis. Apoptosis can be initiated by three different pathways in mammals: the extrinsic pathway, the intrinsic pathway, or the granzyme B pathway50. Caspase-3 is the main executioner protein in apoptotic cells as it can be activated through both extrinsic and intrinsic signaling pathway51. In immunoblot analysis, O. japonicus DW and EtOH extracts significantly increased Caspase-3 activation in a dose- and time-dependent manner (Fig. 11). The result suggests that O. japonicus extracts induce caspase-dependent apoptosis by regulating pro- or anti-apoptotic genes, which can lead to increase of Caspase-3 activation.
In conclusion, O. japonicus decreased cell viability, increased apoptosis rate in both ELISA assay and flow cytometry analysis, and regulated mRNA expression of apoptosis-related genes Bcl-2, Mcl-1, Bax, Survivin, and Cyclin D1. The effect on the p21 gene is uncertain. O. japonicus also increased Caspase-3 protein activation. DW extracts produced more significant effects than EtOH extracts in the cell viability assay, FACS analysis, and real-time PCR analysis. Particularly, treatment with DW extract at 300 μg/ml dose for 72 h produced the most significant effects. These results taken together, indicate that O. japonicus DW and EtOH extracts show anti-tumor effects on SNU-1079 cells through inhibition of cell proliferation and induction of apoptosis via the mitochondria-mediated intrinsic pathway which leads to Caspase-3 activation. Since SNU-1079 is iCCA cell line, O. japonicus is revealed to be a possible therapeutic herbal medicine for iCCA. Further investigations are required to define the molecular mechanisms through which O. japonicus induces apoptosis, via the extrinsic pathway and the granzyme B pathway. Investigation to confirm the hypothesis that O. japonicus functions by regulating G1-G2-M cell cycle progression, also needed.